Pharmaceuticals & logistic mechanisms, Grace Adeye (SPS)
advertisement

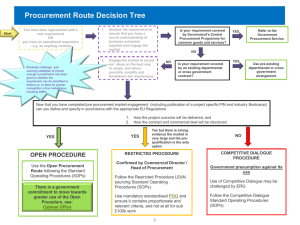
Management of Medicines and Pharmaceutical Supplies for use in the prevention and treatment of Preeclampsia and Eclampsia Grace Adeya, SPS/MSH February 23, 2011 Why Consider Medicine and Pharmaceutical Supplies Management Issues? Effective management of PE/E helps ensure that medicines and supplies are on hand for immediate administration. Effective management requires careful product selection, procurement, storage, distribution, and use. PE/E medicines are in many countries part of the national pharmaceutical supply system Subject to the same structural, financial and human resource constraints as all products that rely on this supply system Pharmaceutical Management Cycle Selection Use Management Support Procurement Distribution Policy and Legal Framework Selection Build consensus on protocol for PE/E with committee of experts and consult best practices Use the following selection criteria At what level of health system? Who will use them? Types of medicines? First-line medicine? Cost Safety and efficacy Quality and stability (storage conditions) Availability for procurement Registered for use in country? Include selected PE/E in national essential medicines list (EML) and standard treatment guidelines (STGs) Selection Use Management Support Procurement Distribution Policy and Legal Framework Selection: Who is making the purchasing decisions at the health facility level? Background of personnel managing medicines at health facility level DRC (N = 30) 7 % pharmacy technicians 80% Nurses Mali (N=100) 18% - pharmacists 3% - pharmacy technicians 4.8% - matron/nurse/midwife 22% - no training Percentage of respondents trained in the management of uterotonic / PE/E medicines 27% 21% 0% 45% 41% 28% 90% 65% 14% 2% 37.3% 15% Proportion of Facilities with a copy of the NEML Percentage of respondents who know MgSO4 is in the NEML Percentage of respondents who know Oxytocin is in the NEML Percentage of respondents who know Calcium Gluconate is in the NEML Percentage of respondents who know Diazepam is in the NEML Procurement Selection Use Management Support Procurement Distribution Policy and Legal Framework Quantity needed Cost Quality: packaging, cold chain Shelf life Supplier performance Management information system (MIS) to monitor consumption Medical Supplies e.g. BP machines Procurement: Staff knowledge and use of essential data for quantification of requirements Knowledge of the stock on hand Knowledge of the quantity dispensed per day Knowledge of the facility’s consumption for one month Knowledge of when and how the medicines were used Ability to use the recorded data for retrospective analysis DRC (N = 30) Mali (N=100) 70% 58% 53% 5% 47% 4% 33% 7% 10% 10% The Procurement Cycle Review Drug Selections Collect Consumption Information Determine Quantities Reconcile Needs and Funds Distribute Drugs Choose Procurement Method Make Payment Locate and Select Suppliers Receive and Check Drugs Specify Contract Terms Monitor Order Status Selection: Cost and Product versatility issues Year Product Name 2007 2008 2009 Average Diazepam 5 mg/ml (general anticonvulsant/antiepileptic; generalized anxiety; pre-operative) Average of Supplier (US$) $ 0.06 $ 0.07 $ 0.07 $ 0.07 Average of Buyer (US$) $ 0.08 $ 0.10 $ 0.06 $ 0.08 Magnesium Sulfate 500 mg/ml (PE/E anticonvulsant ) Average of Supplier (US$) $ 0.09 $ 0.10 $ 0.09 $ 0.09 Average of Buyer (US$) $ 0.17 $ 0.17 $ 0.17 $ 0.17 Source: MSH International Drug Price Indicator Guide Distribution and Inventory Management Effects of heat and light Cold chain equipment and transportation Cold box or packs Refrigerators Excursion? Selection Use Inventory monitoring system Management Support Procurement Distribution Policy and Legal Framework Stock cards and registers Distribution network and transportation Vertical vs. Integrated (How do PE/E products fit into overall supply system?) Delivery kit system? Non-facility locations Distribution: Storage Conditions DRC (N = 30) Mali (N=60) Stock/storage location is secure (locked door, wire mesh on the windows, locked cabinets) 83% 87% Storage location is visibly free of harmful insects and rodents 83% 83% Products are arranged well on shelves or pallets 78% 92% Products are arranged so that identification labels and expiration or manufacture dates are visible 67% 82% Products are stored and organized according to expiration dates (FEFO) 67% 85% Boxes and products are in good condition 94% 87% Boxes and products are protected from water and moisture 94% 80% Products are protected from direct light and sun at all times 94% 88% The store has operational refrigerators 28% 43% The temperature of the cold chain is recorded and monitored regularly 0% 7% Temperatures of the cold chain are between 2°C and 8°C 0% 3% Storage Conditions Use Policy: Who is allowed to prescribe MgSO4? Training in PE/E management: What skills are needed? Service delivery protocols Indications Dose Contraindications Management of side effects Skilled birth attendants Client counseling Adverse drug reaction monitoring Selection Use Management Support Procurement Distribution Policy and Legal Framework USE: Respondents Knowledge of Recommended Treatment DRC (N = 30) Mali (N=100) Percentage of respondents who know Oxytocin is the recommended medicine for the practice of AMSTL 90% 56% Percentage of respondents who know MgSO4 is the recommended medicine for the management of PE/E 8% 23% USE: Product Availability DRC (n = 18) - Mali (n = 60) 46% Oxytocin 10 IU/ml ampoule 78% 34% Magnesium sulfate 4 g ampoule 11% 10% Magnesium sulfate 2 g ampoule - 9% Calcium gluconate 10 mg ampoule 22% 15% Diazepam 10mg inj 56% - Medicines Available Oxytocin 5 IU/ml ampoule Management Support Standard Operating Procedures Financing Information management (MIS) Human resources Preservice education Continuing education In-service education Monitoring and supervision Selection Use Management Support Procurement Distribution Policy and Legal Framework Management Support: Advocacy for Maternal Health: Maternal health issues should always be included among the health priorities. Are maternal health program personnel at the table when decisions are being made on priorities for procurement Forum for improved and regular communication between doctors, midwives and pharmacists e.g Drug and Therapeutics Committees Improved selection, quantification and ordering of uterotonics Clarification of roles and responsibilities of pharmacy, delivery room and recovery ward personnel Policy and Legal Framework Selection Management Support Use Procurement Distribution Policy and Legal Framework EML and STG Registration issues Importation Centralized vs. decentralized; vertical vs. integrated programs Financing mechanisms: cost recovery, cost sharing, insurance Pharmacovigilance PE/E service delivery protocols Human resources: who is authorized to prescribe and dispense? Thank you