PowerPoint - Q-CROC
advertisement

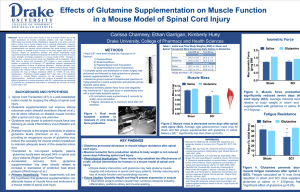
Tumor Metabolism and Chemotherapy Resistance Glutamine a Paradox Michael B. Sawyer, MD, B.Sc. Phm. Associate Professor, Division of Medical Oncology FRCPC (Int. Medicine & Medical Oncology) Dip.ABIM (Int. Medicine & Medical Oncology) Dip.American Board of Clinical Pharmacology Fellow American College of Clinical Pharmacology Potential Conflict of Interest • Dr. Michael Sawyer – Abraxis, research funding (2006-present) – AstraZeneca, research funding (2006-present) – Roche research funding (2005-present) Outline • In vitro glutamine research • In vivo glutamine research • Clinical glutamine research – Oxaliplatin neuropathy and glutamine • Future Research – Translational research into glutamine and oxaliplatin What is Glutamine • • • • In health non essential amino acid Conditionally essential amino acid in illness Preferred fuel for enterocytes and immune cells Precursor for glutathione – major cellular antioxidant • Involved in interorgan nitrogen exchange and maintenance of pH homeostasis Altered Metabolism in Cancer Cells • Warburg noted aerobic gycolysis in 1927 • Glutamine similarly appeared to be metabolized inefficiently • Many cancer cell lines appear to have a “glutamine” addiction – Withdrawal of glutamine causing cell death – Withdrawal of glutamine and substitution of alpha ketoglutarate rescues the cells from death but does not allow proliferation Altered Metabolism in Cancer Cells • Activation of the oncogene Myc activates many enzymes in glutamine metabolism – Glutamine transporters SLC38A5 and SLC1A5 – Glutaminase that converts glutamine to glutamate releasing free ammonia • Glutamine replenishes alpha ketoglutarate • Provides NADPH for biosynthetic reactions • Provides nitrogen for synthesis of non essential amino acids Putative in vitro role of gln In Vivo Glutamine Models • Surgeons and radiation oncologists have not had these concerns • Klimberg, Rombeau and Coupland have studied glutamine in multiple in vivo models • Radiation immunotherapy and methotrexate • Demonstrated increased natural killer cell activity, decreased tumor glutathione, increased efficacy and decreased toxicity Hypothesis • Postulated that glutamine supplementation would improve the therapeutic index of irinotecan • Glutamine would decrease reduced glutathione in tumor cells • Glutamine would increase reduced glutathione in normal tissues such as the intestine decreased chemo toxicity • Gln would not increase heat shock proteins in the tumor • Gln would increase heat shock proteins in normal tissues • Overall glutamine would increase the efficacy of chemotherapy in the tumor and decrease the toxicity of chemotherapy in the normal tissues Dietary treatments • Treatment groups Control diet Prebiotic enriched diet Fish oil enriched diet Bolus glutamine + irinotecan 125mg/kg×3days N=8-12 for each treatment group • Timeline -28 Control, prebiotic, fish oil diets start irinotecan 1, 2, 3 Glutamine bolus Day Comparison of Effects of Different Dietary Treatments on Irinotecan-Induced Diarrhea diarrhea scoring 3.5 control diet + irinotecan 3 Gln bolus + irinotecan 2.5 prebiotics + irinotecan fish oil + irinotecan 2 1.5 1 0.5 0 Time (day) 0 2 4 6 8 10 Comparison of Effects of Different Dietary Treatments on Irinotecan-Induced Diarrhea Incidence of severe diarrhea 0.7 incidence 0.6 0.5 * 0.4 0.3 0.2 0.1 0 control Gln bolus prebiotic fish oil Does Glutamine Treatment Potentiate HSP Response in Tumor as Well? Control diet Gln bolus + + irinotecan + irinotecan Hsp70 Hsp27 control diet+irinotecan Gln bolus+irinotecan control diet+irinotecan Gln bolus+irinotecan Hsp90α Gln Bolus vs. Control- Colonic Mucosa Control diet + irinotecan Gln bolus + irinotecan * * Hsp70 contro diet+irinotecan Gln bolus+irinotecan * * Hsp27 control diet+irinotecan Gln bolus+irinotecan * Hsp90α * control diet+irinotecan Gln bolus+irinotecan Hsc70 control diet+irinotecan Gln bolus+irinotecan Dietary Effects on Irinotecan/5FU Efficacy Dietary Effects on Normal Tissue GSH/GSSG Dietary treatment total GS H* GS S G re du ce d GS H GS H/GS S G REF 1.86±0.12 0.014±0.0021 1.83±0.12 135.59±12.6 CON 1.65±0.057 0.033±0.011 1.58±0.054 ¤ 67.66±17.59 GLN 1.57±0.097 0.012±0.001 1.55±0.097 136.22±13.33 FO 1.72±0.12 0.015±0.003 1.69±0.11 GLN/FO 1.74±0.062 0.0153±0.0016 1.71±0.062 117.92±13.23 0.772 0.047 0.950 0.041 0.181 0.162 0.133 0.192 0.613 0.041 0.782 0.016 Glutamine 124.33±12. 97 2-way N-3 fat ty ANOVA acids p value Interaction Dietary Effects on Tumor Tissue GSSG/GSH Dietary treatment total GS H GSSG re du ce d GSH GSH/GSSG CON 1.56±0.095 0.017±0.0029 1.52±0.090 98.61±14.86 GLN 1.2±0.17 0.024±0.0043 1.15±0.16 54.97±10.05 FO 1.26±0.14 0.023±0.0045 1.22±0.14 58.59±11.03 GLN/FO 1.096±0.12 0.028±0.0056 1.04±0.12 47.17±12.52 0.090 0.231 0.073 0.035 0.188 0.264 0.160 0.063 0.529 0.787 0.511 0.197 Glutamine 2-way N-3 fat ty ANOVA acids p value Interaction Wang et al. • 86 metastatic colorectal cancer patients • Randomized to glutamine (n=42) or control group (n=44) • Treated with oxaliplatin (85) mg/m2 days 1 and 15 and weekly 5-flurouracil (5-FU; 500 mg/m2) and folinic acid (FA 20 mg/m2) on days 1, 8, and 15 • Glutamine was administered 15 g twice a day for 7 days every two weeks Incidence of oxaliplatin-induced neuropathy in different patient groups Wang, W.-S. et al. Oncologist 2007;12:312-319 Outcome of oral glutamine supplementation Wang, W.-S. et al. Oncologist 2007;12:312-319 Table 4. Response to oxaliplatin-based chemotherapy in different patient groups Wang, W.-S. et al. Oncologist 2007;12:312-319 Copyright ©2007 AlphaMed Press Survival curves of metastatic colorectal cancer patients receiving (filled circle) or not receiving (open circle) glutamine supplementation during oxaliplatin treatments plotted by the Kaplan-Meier method (p = .788; log-rank test) Wang, W.-S. et al. Oncologist 2007;12:312-319 Wang Study Interpretations • • • • • Hard endpoint: survival Not placebo controlled No pharmacokinetics Did not look at diarrhea No mechanism of action – No glutathione levels – No HSP • Started the day of chemotherapy Sanofi’s Glutox Study • Using FOLFOX • Randomized placebo controlled • Starting glutamine/placebo two days before chemotherapy • Looking at diarrhea Cross Cancer Parallel Glutox • Patients randomized to glutamine first cycle or second cycle • Oxaliplatin pharmacokinetics done in cycle 1 and 2 • Measurement of plasma, red blood cell glutathione (GSSG and GSH) levels in cycle 1 and 2 • Measurement of white blood cell heat shock protein levels Take Home Messages • • • • • Translational research matters In vitro models may not hold up in in vivo models In vitro models may not hold to human clinical trials Cancer cells are model systems An isolated cancer cell is not a syngenic tumor graft nor a human cancer patient Acknowledgments • Hongyu Xue MD PhD • Co-Principal Investigators: Dr. Vickie Baracos Dr. Catherine Field Dr. Leo Dieleman Alberta Health Services Cancer Care Lab members: Abha Hoedl Michelle Mackenzie Dr. Marina Mourtzakis Stephanie Best Sue Goruk Celine CCI vivarium staff Glutamine Effects on -Glucuronidase vs. control diet + irinotecan Stress response study