Oscar-Della-Pasqua-P..
advertisement

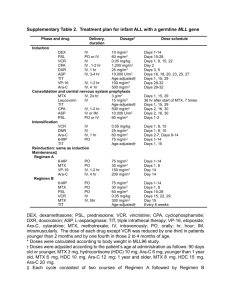
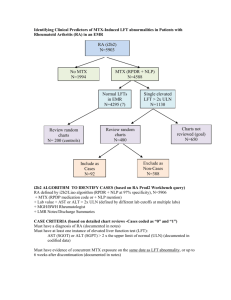
Personalised Medicine: Beyond the buzzword Dr. Oscar Della Pasqua Clinical Pharmacology GlaxoSmithKline, United Kingdom Outline - Does ‘personalised’ effectively mean the same for clinicians, patients and industry? - What are the implications for drug development ? Effectiveness – integrated measure(s) of efficacy and safety shift in paradigm from ‘one dose fits all’ shift in paradigm from ‘one endpoint fits all’ shift in paradigm from ‘large, non-enriched trials’ - Model-based approach to integrate data: right dose, right patient, right methods - Conclusions ‘Clinical Reality…. We’ve got a new wonder drug! - But I wonder which what itdose will do to prescribe. for you. Treatment Decisions All patients with same diagnosis 1 Non-responders or toxic responders Treat with alternative drug 2 Responders and patients not predisposed to toxicity Treat with most suitable dose Biomarkers of Drug Response Response Clinical Relevance - Predictive Value Utility of the information/biomarker Best Y N Good Y Poor N N Variation Y N Y Y N Variation Examples – – – – Not a biomarker ErbB-2 over-expression and response to Herceptin ALOX5 promoter in asthma CrCL Bone marrow density N Y CYP2D6 - Polymorphisms • Number of functional CYP2D6 alleles (0 - 13) determines concentrations of nortriptyline. 2 allele patients had greater clearance than 1 or 0 allele patients. Conc (nmol/L) 60 • Lack of efficacy in CYP2D6 x 13 patients Nortriptyline 25 mg dose Number of functional CYP2D6 genes 0 1 30 2 3 13 0 0 24 48 Hours 72 Clinical relevance of CYP2D6 Nortriptyline dosing recommendation in Europe Clinical Relevance of CYP2D6 Strattera - No Dosing Adjustment Initial approval 2002, USA Are the answers to personalised medicine really here or does one need to look beyond? (Am J Psychiatry 2002; 159:122–129) Consider Genetics Disease / Pharmacokinetics / Pharmacodynamics C G A G C C T A C A T A C T A A T A C C T G A C A C G A G C C T G A C C T T C A A T G G A T A G A A T T C A A A G T A G A T A C G A T G A A T G G A A T A G A T T A A G G T T A C C A T A A G C C T A A G G T A T A T A A C C T A T T G C A G C A A T A G T Epidemiology / Genetics / Clinical Pharmacology Can one predict the impact of variability or noise in drug effect with a single marker? What do you see when you have spent 8 months designing a sports car? Consider Intrinsic and Extrinsic Factors Disease / Pharmacokinetics / Pharmacodynamics C O M E D P R O T E I G E N E B I N D I I N T A R G E D I C A T I O N S E T A B O D R Y T F R A A C N T S T I C P O L Y M O R P R O S R T S E A S E S E R S T S H E Y W H I E D R U G M E T A B O L I S I N G B A L S I G H T M S Z Y M E S E C E P T O R S Epidemiology / Genetics / Clinical Pharmacology Model-based Approaches for Prediction of Response Disease / Pharmacokinetics / Pharmacodynamics C O M E D P R O T E I G E N E B I N D I I N T A R G E D I C A T I O N S E T A B O D R Y T F R A A C N T S T I C P O L Y M O R P R O S R T S E A S E S E R S T S H E Y W H I E D R U G M E T A B O L I S I N G B A L S I G H T M S Z Y M E S E C E P T O R S Epidemiology / Genetics / Clinical Pharmacology BeSt study design • • Retrospective, multi-centre, open 509 patients with active RA enrolled in this study are participants in a trial to test the effectiveness of different treatment strategies (BeSt- study) • all patients have active disease according to ACR criteria, disease duration < 2 years • 247 patients are treated with monotherapy MTX Wessels et al. Arthritis Rheum.56:1765-75, 2007 BeST study: summary DAS >2.4 205 RA patients Active RA at baseline DAS 4.5 DAS 2.4 MTX 15 mg/week or 25 mg/week, folic acid 1 mg/day RESPONSE 47% at 6 months ADVERSE DRUG EVENTS 30% age gender hormonal status co-morbidity ethnicity previous DMARD use HLA-DRB1 alleles (shared epitope) PTPN22 Cytochrome P450 enzymes Candidate genes Genes Disease duration disease activity Anti-CCP rheumatoid factor Host Disease Life style e.g. smoking and diet social class Environment Drug Treatment outcome Factors influencing outcome Disease activity score ACR criteria Health assessment questionnaire Radiographic score Measures to evaluate outcome Folate pathway RFC MTX response MTHFR haplotype as factor for 100 88 80 77 66 62 46 46 50 good clinical response MTHFR testing may determine which RA patients will benefit from MTX good clinical improvement ie 2 co p ie s co p 1 0 co p ie s moderate clincal imrpovement Number of MTHFR 677C1298A haplotype copies genetics contribute to MTX treatment outcome in RA ‘Adenosine release’ Good clinical response with MTX at 6 months (%) AMPD1 T-allele, ATIC CC genotype, ITPA CC genotype are 2-3 fold more likely to achieve good clinical response 93 50 58 60 61 68 75 37 26 42 37 41 ITPA 43 43 AMPD 47 Current MTX pharmacogenetic research From associations with genes to a predictive clinical tool “MTX sensitive RA” - Simple model - validation in 2nd cohort Development of a predictive model of clinical response 24 baseline variables believed to influence RA disease state and MTX drug response were selected based on literature RFC 17 SNPs in 13 genes involved in the MTX mechanism of action, purine and pyrimidine synthesis ITPA AMPD Factors determining efficacy for individual MTX monotherapy Baseline Variable Gender Score Female premenopausal postmenopausal Male Disease activity DAS at baseline 3.8 ≤ but DAS at baseline >3.8, DAS at baseline >5.1 5.1 ≤ 1 1 0 0 3 3.5 Immunological factors Rheumatoid factor negative and non-smoker Rheumatoid factor negative and smoker Rheumatoid factor positive and non-smoker Rheumatoid factor positive and smoker 0 1 1 2 Genetic factors MTHFD1 1958 AA genotype AMPD1 34 CC genotype ITPA 94 A- allele carrier ATIC 347 G-allele carrier Other genotypes 1 1 2 1 0 Suggestions for clinical application of the model Categories Clinical consequence Scores ≥ 6 Low probability to respond to MTX monotherapy. Consider a combination strategy. Scores < 6, but > 3.5 Intermediate probability to respond to MTX monotherapy. Evaluate after 3 months therapy. Scores ≤ 3.5 High probability to respond to MTX monotherapy Dose escalation to 25 mg/week if necessary. Receiver Operating Curves (ROC) 1,0 0,8 sensitivity 0,5 PG Model: pharmacogenetic model True positive response 95% 0,3 (36 out of 38) non-genetic model Non-genetic model True negative response 87% (62 out of 72) Percentage of patients categorised: 32% Percentage of patients categorized: 60% 0,0 0,3 0,5 1- specificity 0,8 1,0 Conclusions - BeST The chance to achieve clinical response with MTX treatment is predictable in recent onset RA. It is feasible to assist initial treatment decisions to tailor therapy in RA patients according to their baseline criteria (symptoms, signs and genotype) Model-based Approaches for Dose Optimisation Disease / Pharmacokinetics / Pharmacodynamics C O M E D P R O T E I G E N E B I N D I I N T A R G E D I C A T I O N S E T A B O D R Y T F R A A C N T S T I C P O L Y M O R P R O S R T S E A S E S E R S T S H E Y W H I E D R U G M E T A B O L I S I N G B A L S I G H T M S Z Y M E S E C E P T O R S Epidemiology / Genetics / Clinical Pharmacology New Technologies – Old tools? From Biomarker data to Treatment Decision JAMA, 296 (12), 2006 The concentration-response surface: Efficacy What is the surface for a given population /patient group? Where are you during development? Multidimensional Diseases - Multiple Endpoints 1. Migraine (4) 2. Alzheimers (2) 3. Acute Pain (3) 4. Lower Back Pain (3) 5. Sleep Disorders (3 or 6) 6. RA (4) 7. OA for symptom modif. (2) 8. Asthma, COPD (2) 9. ED (3) 10. Skin Aging (2) 11. Menopausal Symptoms (3) 19. Organ Transplantation (2) 12. Fracture Healing (2) 20. Primary Biliary Cirrhosis (4) 13. Acne (4) 21. BPH (2) 14. Male Pattern Baldness (2) 22. Multiple Sclerosis (2) 15. Glaucoma (9) 23. Epilepsy (3) 16. 17. Ophthalmology – dry eye (2) 24. 25. Hepatitis B (up to 3) 18. Vaginal Atrophy (3) 26. Vaccines (up to 23) Operable Breast Cancer (with + auxiliary lymph nodes) (2) Fibromyalgia (2-3) Model-based risk assessment Model-based risk assessment Model-based Approaches: Dosage strategy for enoxaparin Observed vs. population predicted anti-Xa concentrations for the two-compartment model with CrCL and weight covariates in the model. Individual data points are shown as dots and the unity as a solid line Three-dimensional surface showing the relationship between CrCL, weight and predicted Css. The surface shows how the Css changes with both weight and CrCL simultaneously Feng et al (2007), Br J Clin Pharmacol 62:165–176 general medical unit 8.3 IU/ kh/h 5.8 IU/kg/h 5.0 IU/kg/h 4.2 IU/kg/h % Css out of range % Css >1.2 UI/ml % Css < 0.5 UI/ml intensive care unit (1, CrCL <30 ml min−1; 2, CrCL 30–50 ml min−1; 3, CrCL >50 ml min−1). Model-based Dose Recommendations Barras et al. (2007) Clin Pharmacol Ther advance online publication doi:10.1038/sj.clpt.6100399 Sotalol in SVT Sotalol conc (ug/mL) Probability of arrhythmia suppression in the 15 children with supraventricular tachycardia vs sotalol trough concentration under steady-state conditions and an 8-h dosing interval. Filled circles 6 neonates (28 days). Effect of Age on Clearance Sotalol oral Clearance (ml/min/kg) Probability of Response PK/PD relationship Age (years) Measured (closed diamonds) and model predicted oral sotalol clearance based on body weight (open diamonds). Median (solid line) and the 10th and 90th percentile (dashed line) of 1,000 simulated data sets. Dose Recommendation Age-specific Dosing regimen for sotalol in children with SVT Black box plots and hatched bars indicate recommended dosing range. (A) Simulated sotalol trough concentrations (125 patients per group and dose level) for paediatric patients with supraventricular tachycardia. Lines indicate 50% and more than 95% efficacy. (B) Patient fraction with 50% and more than 95% probability of arrhythmia suppression. Arrows indicate start and target doses. Summary - Does ‘personalised’ effectively mean the same for clinicians, patients and industry? - What are the implications for drug development ? Effectiveness – integrated measure(s) of efficacy and safety shift in paradigm from ‘one dose fits all’ shift in paradigm from ‘one endpoint fits all’ shift in paradigm from ‘large, non-enriched trials’ - Model-based approach to integrate data: right dose, right patient, right methods - Conclusions Personalised Treatment: Delicate Balance Between Benefit and Risk The greatest obstacle to discovery is not ignorance, but the illusion of knowledge by Daniel Boorstin