Breakthrough Therapies, Jeff Allen, PhD
advertisement

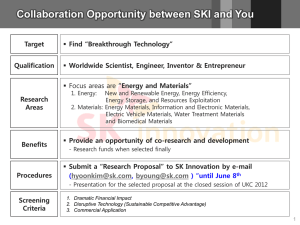
Expediting Drug Development: Breakthrough Therapies Jeff Allen, PhD Executive Director, Friends of Cancer Research Courtesy of the American Association of Cancer Research 2011 Cancer Progress Report The New York Times September 18, 2010 New Drugs Stir Debate on Rules of Clinical Trials By AMY HARMON “But critics of the trials argue that the new science behind the drugs has eclipsed the old rules — and ethics — of testing them. They say that in some cases, drugs under development, PLX4032 among them, may be so much more effective than their predecessors that putting half the potential beneficiaries into a control group, and delaying access to the drug to thousands of other patients, causes needless suffering.” Getting Breakthrough Therapies to Patients • The 2011 Conference included a panel entitled: Development Paths for New Drugs with Large Treatment Effects Seen Early. • The workgroup proposed scientific strategies to ultimately expedite FDA approval for a drug showing dramatic responses in the early stages of development while maintaining drug safety and efficacy standards. Goals of Breakthrough Therapy Designation Goal 1: Expedite drug development process for products that show remarkable clinical activity early Goal 2: Minimize the number of patients exposed to a potentially less efficacious treatment Concept Scientific Whitepaper Bipartisan Legislative Solution Tool in use by FDA to expedite drug development Breakthrough Therapies “(a) Designation of a Drug as a Breakthrough Therapy.— “(1) IN GENERAL.—The Secretary shall, at the request of the sponsor of a drug, expedite the development and review of such drug if the drug is intended, alone or in combination with 1 or more other drugs, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on 1 or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. (In this section, such a drug is referred to as a ‘breakthrough therapy’.) “(2) REQUEST FOR DESIGNATION.—The sponsor of a drug may request the Secretary to designate the drug as a breakthrough therapy. A request for the designation may be made concurrently with, or at any time after, the submission of an application for the investigation of the drug under section 505(i) or section 351(a)(3) of the Public Health Service Act. “(3) DESIGNATION.— “(A) IN GENERAL.—Not later than 60 calendar days after the receipt of a request under paragraph (2), the Secretary shall determine whether the drug that is the subject of the request meets the criteria described in paragraph (1). If the Secretary finds that the drug meets the criteria, the Secretary shall designate the drug as a breakthrough therapy and shall take such actions as are appropriate to expedite the development and review of the application for approval of such drug. Breakthrough Therapies “(B) ACTIONS.—The actions to expedite the development and review of an application under subparagraph (A) may include, as appropriate— “(i) holding meetings with the sponsor and the review team throughout the development of the drug; “(ii) providing timely advice to, and interactive communication with, the sponsor regarding the development of the drug to ensure that the development program to gather the non-clinical and clinical data necessary for approval is as efficient as practicable; “(iii) involving senior managers and experienced review staff, as appropriate, in a collaborative, cross-disciplinary review; “(iv) assigning a cross-disciplinary project lead for the Food and Drug Administration review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor; and “(v) taking steps to ensure that the design of the clinical trials is as efficient as practicable, when scientifically appropriate, such as by minimizing the number of patients exposed to a potentially less efficacious treatment.”; Courtesy of AACR Annual Meeting 2013 Proposed Criteria for Breakthrough Therapy Designation Category Qualitative Criteria 1. No standard of care (SoC); serious condition with poor outcome • Unprecedented early activity in Phase I: CRR*, ORR* or CBR* • Acceptable safety 2. Substantial efficacy improvement over well characterized SoC; serious condition with poor outcome • Exceptional early activity based on response rates (CRR, ORR) and durability or disease control • Acceptable safety 3. Substantial therapeutic index advantage over a well characterized SoC; serious condition with poor outcome • Superior safety/tolerability combined with superior or clearly maintained efficacy http://www.focr.org/sites/default/files/CCCR12Breakthrough.pdf *CR=complete response, ORR=overall response rate, CBR=clinical benefit rate Current Breakthrough Therapies Breakthrough Therapy Designation Requests Requests Received Requests Granted Requests Denied CDER (as of 6/14/2013) 58 21 16 CBER (as of 5/31/2013) 6 0 5 FDA Total 64 21 21 Implementation of Breakthrough Designation May Mar/Apr LDK378 Jan/Feb: Kalydeco receives two designations July 2012: Legislation signed into law Ibrutinib given two designations Palbociclib Ibrutinib (3) Lambrolizumab Daclatasvir SD101 announce Breakthrough designations Daratumumab ABT-450 obinutuzumab Selbelipase alfa Asfotase alfa announce Breakthrough designations 12 Breakthrough Designations Announced By Companies So Far • Kalydeco (Vertex) - 2 cystic fibrosis designations (combination and monotherapy) • Ibrutinib - (J&J/Pharmacyclics) - 3 designations (mantle cell lymphoma, WM, CLL) • LDK378 - (Novartis) – ALK+ NSCLC resistant to crizotinib • palbociclib - (Pfizer) - ER+, HER2- locally advanced or metastatic breast cancer • lambrolizumab – (Merck) – inoperable and metastatic melanoma • Daclatasvir – (BMS) – Hepatitis C in combination with two other direct-acting antivirals • Daratumumab - (Janssen) - multiple myeloma after three prior lines of therapy • SD101 - (Scioderm) - topical cream for the treatment of inherited Epidermolysis Bullosa (EB) • ABT-450 - (AbbVie) - interferon-free 3-DAA treatment of Hepatitis C in combination • Obinutuzumab - (Genetech) - Chronic Lymphocitic Leukemia (CLL) • Sebelipase Alfa - (Synageva) - early onset lysosomal acid lipase deficiency (LAL deficiency) • Asfotase Alfa - (Alexion Pharmaceutical) - hypophosphatasia (HPP) whose first signs or symptoms occurred prior to 18 years of age, including perinatal-, infantile-, and juvenile-onset forms of the disease. Characteristics of Breakthrough Therapies Kalydeco - cystic fibrosis transmembrane conductance regulator (CFTR) potentiator • Data: Phase 2 clinical trial of Kalydeco and VX-809 in multiple combinations showed significant improvements in lung function • Phase 3 trial scheduled to be half the duration of original phase 3 • Note: Combination drugs; supplemental indication; functional endpoint ibrutinib - Specifically target and selectively inhibit BTK • Data: 12-month median progression-free survival is 93% • In patients for whom a bone marrow biopsy was done, tumor infiltration decreased by 82 percent • Note: No current treatment (WM); substantial improvement over SoC (CLL) LDK378 – selective inhibitor of ALK • Data: Phase 1 – 80%RR in patients who progressed following crizotinib • Note: Substantial improvement over SoC; second-in-class palbociclib - Cyclin-dependent kinases (cdk) 4 and 6 inhibitor • Data: Phase 2 – PFS = 26.1mo vs 7.5mo letrozole alone • Note: Substantial improvement over SoC; surrogate endpoint lambrolizumab– PD-1 inhibitor • Data: Phase 1B – 51% of patients showed an objective anti-tumor response; 9% showed complete response after the 12-week assessment • Note: Current SoC isn’t effective for some patients; relatively low incidence of high-grade adverse events daclatasvir – interferon-free Hepatitis C drug • Data: Phase 2a - Stopped the virus from replicating in 15 of 16 patients during a 24 week trial • Note: Combination drugs; patients resistant to SoC