Update on Anti-VEGF Therapy in
Diabetic Macular Edema, Neovascular
AMD, and Retinal Vein Occlusion
Jonathan S. Chang, MD
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, Florida
A REPORT FROM THE 2012 ANNUAL MEETING OF THE
ASSOCIATION FOR RESEARCH IN VISION AND OPHTHALMOLOGY
© 2012 Direct One Communications, Inc. All rights reserved.
1
A New Focus on Ophthalmic Disease
Use of anti-vascular endothelial growth factor (antiVEGF) agents has become a mainstay in treating
several common retinal diseases.
This drug class initially was used to manage
neovascular age-related macular degeneration
(AMD).1–3
Its value recently was affirmed for treating both
branch retinal vein occlusion (BRVO)4 and central
retinal vein occlusion (CRVO).5
Further clinical data support their use in managing
diabetic macular edema (DME).6
© 2012 Direct One Communications, Inc. All rights reserved.
2
Novel Drugs for Ophthalmic Indications
Ranibizumab has been approved by the US Food and
Drug Administration (FDA):
» To treat patients with neovascular AMD
» For macular edema following retinal vein occlusion (RVO)
» For DME (August 2012)
Aflibercept (VEGF Trap-Eye) is approved by the FDA
for treating neovascular AMD.
Intravitreal bevacizumab is widely used off-label for
the treatment of these conditions, especially
neovascular AMD.
© 2012 Direct One Communications, Inc. All rights reserved.
3
Clinical Trials of VEGF Inhibitors
Several recent studies offered additional insight into
the use of anti-VEGF agents.
The READ,3,7 RISE,8 and RIDE8 trials involved the
use of ranibizumab in patients with DME.
The DA VINCI trial9 demonstrated the effect of
aflibercept therapy in DME patients.
The 2-year results of the CATT trial10 compared use
of bevacizumab with ranibizumab therapy in AMD
patients.
© 2012 Direct One Communications, Inc. All rights reserved.
4
Clinical Trials of VEGF Inhibitors
Investigators involved in the VIEW 1 and VIEW 2
studies11 reported on the use of aflibercept in
patients with neovascular AMD.
The management of RVO changed after analysis of
the CRUISE4 and BRAVO5 study results.
Further information has been gathered in the
HORIZON study.12
Questions about the management of patients
diagnosed with retinal disease remain.
© 2012 Direct One Communications, Inc. All rights reserved.
5
Issues Involved in Using VEGF inhibitors
Monthly versus as-needed dosing
The choice of an anti-VEGF agent for treating each
condition
Corticosteroid and laser therapy in patients affected
by RVO and DME
The safety of intravitreal injections of VEGF
inhibitors
© 2012 Direct One Communications, Inc. All rights reserved.
6
Diabetic Macular Edema
© 2012 Direct One Communications, Inc. All rights reserved.
7
Diabetic Macular Edema:
DRCR Trial
In the Diabetic Retinopathy Clinical Research
Network (DRCR) protocol I,6,13 investigators
compared sham therapy plus prompt focal laser
treatment with:
» Ranibizumab plus prompt laser therapy
» Ranibizumab plus deferred laser treatment
» Triamcinolone plus prompt laser therapy
Two-year follow-up data recently showed improved
visual acuity and central foveal thickness in both
ranibizumab groups as compared with those
receiving sham plus laser therapy or triamcinolone
plus laser therapy.14
© 2012 Direct One Communications, Inc. All rights reserved.
8
Diabetic Macular Edema:
RISE and RIDE Trials
RISE8 and RIDE8 were two parallel studies
comparing the use of 0.3 or 0.5 mg of ranibizumab
given monthly with sham therapy for DME.
In the RIDE trial, patients receiving 0.3 and 0.5 mg
of ranibizumab gained 8.6 and 9.7 letters of visual
acuity, respectively.
Similar results were reported in the RISE trial, with
patients given 0.3 mg of ranibizumab gaining 10.0
letters and those given 0.5 mg gaining 9.3 letters.
Patients receiving ranibizumab at either dose level
showed improvement in visual acuity and retinal
thickness and a reduction in diabetic retinopathy.
© 2012 Direct One Communications, Inc. All rights reserved.
9
Diabetic Macular Edema:
READ 3 Trial
The READ 3 trial7 compared two different
ranibizumab doses in patients with DME.
Patients were randomized to groups receiving 0.5 or
2.0 mg of ranibizumab monthly for 6 months.
At month 6, patients in the 0.5-mg group gained a
mean of 8.72 letters, whereas those in the 2.0-mg
group gained a mean of 7.35 letters.
At month 12, patients in the 0.5-mg group gained
10.88 letters as compared with the 7.39 letters
gained by the 2.0-mg group (P = 0.03).
© 2012 Direct One Communications, Inc. All rights reserved.
10
Diabetic Macular Edema:
BOLT Study
The BOLT study15 compared the use of bevacizumab
with focal laser therapy in patients with DME.
Patients were randomized to groups receiving either
bevacizumab or macular laser therapy and were
followed for 2 years.
Patients in the bevacizumab group gained a mean of
8.6 letters, whereas those in tThe macular laser
therapy group lost a mean of 0.5 letters.
Central foveal thickness also was reduced in the
bevacizumab group as compared with the laser
treatment group.
© 2012 Direct One Communications, Inc. All rights reserved.
11
Diabetic Macular Edema:
DA VINCI Study
Results from the DA VINCI trial9 demonstrated
improvement in the eyes of patients with DME who
used aflibercept as compared with patients who
underwent focal laser therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
12
Diabetic Macular Edema:
Treatment Considerations
Many ophthalmologists prefer to administer
treatment at regular intervals instead of as needed in
patients affected with a chronic process like DME.
Persistent edema appears to be more common
among patients with DME than in those with
neovascular AMD, even in eyes treated regularly.
The clinician’s threshold for residual edema may be
greater, because the two diseases have different
natural histories.
© 2012 Direct One Communications, Inc. All rights reserved.
13
Diabetic Macular Edema:
Treatment Considerations
Patients with DME tend to be young:
» They may receive a considerable number of injections over
their lifetime.
» Frequent office visits can interrupt their daily lives.
The current VIVID-DME (ClinicalTrials.gov
NCT01331681) and VISTA DME (ClinicalTrials.gov
NCT01363440) trials are examining use of a loading
dose of aflibercept every month for 6 months
followed by dosing every 6 months.
» Data on these phase III trials should be available in the
coming years.
© 2012 Direct One Communications, Inc. All rights reserved.
14
Diabetic Macular Edema:
Corticosteroid Therapy
Corticosteroid therapy continues to be an option for
patients with DME.
However, it tends to be used now in individuals who
do not respond to anti-VEGF treatment.
Increased intraocular pressure and cataracts
continue to be the main adverse effects related to the
use of corticosteroids.
© 2012 Direct One Communications, Inc. All rights reserved.
15
Diabetic Macular Edema:
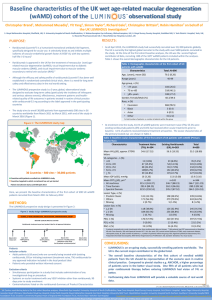
FAME Trial
The FAME trial16 examined the use of a fluocinolone
acetonide implant in patients with DME.
The results demonstrated efficacy with both a 0.2
and 0.5 µg/d implant.
At 2 years, a greater than 15-letter improvement in
visual acuity was achieved in:
» 28.7% of patients receiving 0.2 µg/d of fluocinolone
» 28.6% of patients receiving 0.5 µg/d of fluocinolone
» 16.2% of the sham treatment group (P = 0.002 for each)
62% of patients receiving 0.2 µg/d did not require
treatment for increased intraocular pressure.
© 2012 Direct One Communications, Inc. All rights reserved.
16
Diabetic Macular Edema:
Corticosteroid Therapy
The FDA has not approved the use of fluocinolone
acetonide implants for treating DME because of
insufficient safety data.
Marketing of the implant for this indication is
approved in Europe. In selected patients,
corticosteroid treatment remains an option.
© 2012 Direct One Communications, Inc. All rights reserved.
17
Neovascular AMD
© 2012 Direct One Communications, Inc. All rights reserved.
18
Neovascular AMD:
Clinical Data and Options
AMD is the leading cause of blindness in the United
States and in other developed countries.17
During the past few months, data from several large
trials have impacted treatment options.
» Use of ranibizumab for neovascular (exudative) AMD was
first reported in the MARINA2 and ANCHOR3 studies.
» Frequency of ranibizumab dosing has been examined in the
PIER,18 PrONTO,19 SUSTAIN,20 EXCITE,21 and HORIZON22
trials.
As anti-VEGF treatment alternatives have expanded,
ophthalmologists now have 3 options: ranibizumab,
aflibercept, and off-label bevacizumab.
© 2012 Direct One Communications, Inc. All rights reserved.
19
Neovascular AMD:
The VIEW Trials
Results from the VIEW 1 and VIEW 2 trials, which
compared the use of aflibercept with ranibizumab
therapy for exudative AMD, were reported recently.11
These parallel, noninferiority trials randomized a
total of 1,217 North American patients to one of four
treatment groups:
» 0.5 mg of ranibizumab monthly
» 0.5 mg of aflibercept monthly
» 2 mg of aflibercept monthly
» Three monthly 2-mg aflibercept injections given as a
loading dose followed by 2 mg of aflibercept every 2 months
© 2012 Direct One Communications, Inc. All rights reserved.
20
Neovascular AMD:
The VIEW Trials
In 94%–96% of patients, moderate vision loss was
prevented.
The three aflibercept groups had results that were
noninferior to those observed in the ranibizumab
group.
Patients receiving 2.0 mg of aflibercept monthly
experienced a 10.9-letter gain as compared with an
8.1-letter gain observed in patients given monthly
ranibizumab.11
This difference was statistically significant.
© 2012 Direct One Communications, Inc. All rights reserved.
21
Neovascular AMD:
The VIEW Trials
The VIEW 2 trial enrolled 1,240 patients in Europe,
Asia, and Latin America and had outcomes similar to
those of the VIEW 1 trial.11
Both studies had adverse event rates that were
similar to each other and to those of prior studies of
anti-VEGF therapy.
Aflibercept may be an option in patients who do not
respond well to ranibizumab or bevacizumab.
© 2012 Direct One Communications, Inc. All rights reserved.
22
Neovascular AMD:
The CATT Trial
Two-year results of the CATT trial,10 a study funded
by the National Eye Institute, recently were
presented at the 2012 ARVO meeting.
A total of 1,185 patients with neovascular AMD were
randomly assigned to one of four treatment groups:
» 0.5 mg of ranibizumab monthly
» 1.25 mg of bevacizumab monthly
» 0.5 mg of ranibizumab as needed
» 1.25 mg of bevacizumab as needed
© 2012 Direct One Communications, Inc. All rights reserved.
23
Neovascular AMD:
The CATT Trial
The as-needed groups were seen every 4 weeks and
received injections when:
» New fluid was present on optical coherence tomography
(OCT)
» New or persistent hemorrhage occurred
» Decreased visual acuity or new leakage was seen on
fluorescein angiography
After 1 year, the monthly groups were divided into
monthly and as-needed treatment cohorts for 1 year.
© 2012 Direct One Communications, Inc. All rights reserved.
24
Neovascular AMD:
The CATT Trial
After 2 years of therapy:
» The monthly ranibizumab group demonstrated a gain of 8.8
letters.
» The monthly bevacizumab group gained 7.8 letters.
» The ranibizumab as-needed group gained 6.7 letters.
» The bevacizumab as-needed group gained 5 letters.
© 2012 Direct One Communications, Inc. All rights reserved.
25
Neovascular AMD:
The CATT Trial
Patients receiving monthly injections gained 2.4
letters more than did those given ranibizumab or
bevacizumab as needed (P = 0.046).
Mean retinal thickness on OCT was 29 μm less in the
monthly treatment groups than in patients receiving
ranibizumab or bevacizumab as needed
This difference also was statistically significant.
© 2012 Direct One Communications, Inc. All rights reserved.
26
Neovascular AMD:
The CATT Trial
No evidence of fluid was found on OCT in:
» 45.5% of the monthly ranibizumab group
» 30.2% of the monthly bevacizumab group
» 22.3% of the as-needed ranibizumab group
» 13.9% of the as-needed bevacizumab group
Over 2 years, the mean number of injections in the
monthly ranibizumab and bevacizumab groups was
22.4 and 23.4, respectively.
The as-needed ranibizumab and bevacizumab groups
received a mean of 12.6 and 14.1 injections,
respectively.
© 2012 Direct One Communications, Inc. All rights reserved.
27
Neovascular AMD:
The CATT Trial
Based on the results of the CATT trial, there is a
2.4-letter difference between monthly dosing and
as-needed dosing in patients with neovascular AMD.
The results also affirmed the effectiveness of off-label
bevacizumab in treating neovascular AMD.
It is not known whether differences in results
between groups given monthly or as-needed dosing
would increase further after 2 years.
Given that many patients with neovascular AMD are
treated for longer than 2 years, clinicians are greatly
interested in the long-term effects of therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
28
Neovascular AMD:
The CATT Trial
Geographic atrophy increased in the monthly dosing
groups when compared with the as-needed dosing
groups.
» It is not clear whether this finding is related to improved
visualization of geographic atrophy due to a decreased
amount of fluid or to a process associated with chronic antiVEGF therapy.
» Geographic atrophy is a factor to consider in future trials of
VEGF inhibitors.
» Other imaging may be useful in this regard.
The study also did not address the efficacy of the
commonly used “treat-and-extend” protocol for
administering ranibizumab or bevacizumab.
© 2012 Direct One Communications, Inc. All rights reserved.
29
Retinal Vein Occlusion
© 2012 Direct One Communications, Inc. All rights reserved.
30
Retinal Vein Occlusion:
FDA-Approved Drugs for Treating RVO
In 2009, the FDA approved the use of a
dexamethasone intravitreal implant for the
treatment of macular edema following RVO.
The following year, it approved the use of intravitreal
ranibizumab for the same indication.
In the past, RVO initially would have been treated
with observation.
Now, based on data from the CRUISE and BRAVO
studies,4,5 prompt treatment of RVO with
ranibizumab has become commonplace.
© 2012 Direct One Communications, Inc. All rights reserved.
31
Retinal Vein Occlusion:
Clinical Trials
Investigators involved in the COPERNICUS study23
reported data showing an improvement in macular
edema after 6 months of monthly aflibercept therapy
in patients with CRVO.
Treatment regimens for RVO now typically start with
monthly injections of anti-VEGF agents before
patients are switched to as-needed dosing with
monthly monitoring.
© 2012 Direct One Communications, Inc. All rights reserved.
32
Retinal Vein Occlusion:
Clinical Trials
Based on data from the HORIZON trial,12,22 in which
patients initially enrolled in CRUISE or BRAVO
continued therapy with as-needed treatment, fewer
injections were required after the initial 12 months of
therapy.
Focal laser therapy often is used adjunctively after
5–6 months of initial therapy.
© 2012 Direct One Communications, Inc. All rights reserved.
33
Retinal Vein Occlusion:
Monitoring Retinal Nonperfusion
Patients with CRVO or BRVO who are given antiVEGF therapy have shown differences in central
nonperfusion.
» However, these discrepancies have not been fully evaluated
in clinical trials.
Peripheral retinal nonperfusion also may recover
with anti-VEGF therapy.
» This finding, however, also has not been fully studied.
Retinal nonperfusion often is a precursor to
neovascularization and may be a measure for future
studies.
© 2012 Direct One Communications, Inc. All rights reserved.
34
Retinal Vein Occlusion:
Monitoring Retinal Nonperfusion
Retinal hemorrhage resolution improves with antiVEGF therapy.
» The mechanism for this clearance is not fully understood.
The use of scatter and focal laser photocoagulation
also is important in these patients.
© 2012 Direct One Communications, Inc. All rights reserved.
35
Safety of Anti-VEGF Therapy
© 2012 Direct One Communications, Inc. All rights reserved.
36
Safety of Anti-VEGF Therapy
In recent clinical trials evaluating VEGF inhibition in
patients with DME, exudative AMD, or RVO,
endophthalmitis occurred in about 1% of patients,
about the same frequency as reported in prior
studies.
As previously mentioned, increased geographic
atrophy has been observed in patients with
neovascular AMD receiving monthly injections of
ranibizumab or bevacizumab.10
» However, this finding has not been reported in patients who
were treated with VEGF inhibitors for DME or RVO.
» It is not clear whether this increase in geographic atrophy is
directly related to anti-VEGF therapy.
Systemic adverse37 effects in patients with neovascular
© 2012 Direct One Communications, Inc. All rights reserved.
Safety of Anti-VEGF Therapy
Systemic adverse effects in patients with neovascular
AMD previously were evaluated in the SAILOR
trial.24
In the CATT trial,10 39.9% of the bevacizumab group
and 31.7% of those receiving ranibizumab developed
one or more serious systemic adverse events, a
statistically significant difference (P = 0.004).10
Serious adverse events included:
» Death (6.1% for bevacizumab vs 5.3% for ranibizumab)
» Arterial thrombotic events (5.0% vs 4.7%)
» Venous thrombotic events (1.7% vs 0.5%)
» Hypertension (0.7% vs 0.5%)
© 2012 Direct One Communications, Inc. All rights reserved.
38
Conclusion
© 2012 Direct One Communications, Inc. All rights reserved.
39
Conclusion
The use of anti-VEGF therapy continues to be a
mainstay of medical retina therapy.
Increased use of VEGF inhibitors for retinal diseases
has led to questions about:
» Their efficacy and safety
» Frequency of treatment
» The possible application of “treat-and-extend” therapy
» The potential role of corticosteroid implants in treating
DME and RVO
A better understanding of the VEGF pathways and
ways that VEGF affects normal physiology will
increase our knowledge of these diseases.
© 2012 Direct One Communications, Inc. All rights reserved.
40
Conclusion
In DME, there appears to be a role for anti-VEGF
agents.6–8,13–15
Regular monthly injections of these agents seem to
produce greater benefit than does as-needed dosing.
Recent data from several large AMD trials
demonstrated the efficacy of aflibercept11 and
validated the off-label use of bevacizumab for
neovascular AMD, albeit at the risk of a greater
frequency of serious systemic adverse events when
compared with ranibizumab.10
Monthly dosing also appeared to produce better
outcomes in AMD than did as-needed dosing.10
© 2012 Direct One Communications, Inc. All rights reserved.
41
Conclusion
For RVO, anti-VEGF agents have changed treatment
planning from observation to earlier intervention.
Further investigation is needed to determine longterm treatment strategies for these patients.
Recent trials have not demonstrated any higher
frequency of adverse systemic effects or additional
adverse events from VEGF-inhibitor therapy.
Future studies will supply greater details on the
safety and efficacy of these agents and optimal
dosing schedules.
© 2012 Direct One Communications, Inc. All rights reserved.
42
References
1.
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular agerelated macular degeneration. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. N
Engl J Med. 2004;351:2805–2816.
2.
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration.
N Engl J Med. 2006;355:1419–1431.
3.
Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related
macular degeneration. N Engl J Med. 2006;355:1432–1444.
4.
Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein
occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–1112.
5.
Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal
vein occlusion: six-month primary end point results of a phase III study. BRAVO Investigators.
Ophthalmology. 2010;117:1124–1133.
6.
Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred
laser or triamcinolone plus prompt laser for diabetic macular edema. Diabetic Retinopathy Clinical
Research Network. Ophthalmology. 2010;117:1064–1077.
7.
Do DV, Campochiaro PA, Boyer DS, et al. Six month results of the READ 3 study: ranibizumab for edema of
the macular in diabetes. Presented at the 2012 Annual Meeting of the Association for Research in Vision
and Ophthalmology (ARVO); May 6–10, 2012; Fort Lauderdale, Florida. Abstract 5282.
8.
Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase
III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
9.
Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the DA VINCI study of VEGF Trap-Eye in eyes
with diabetic macular edema. DA VINCI Study Group. Ophthalmology. 2012;119:1658–1665.
10. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular agerelated macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398.
© 2012 Direct One Communications, Inc. All rights reserved.
43
References
11. Heier JS. VEGF Trap-Eye for AMD: VIEW 1/VIEW 2 studies. Presented at the 2011 Annual Meeting of the
American Academy of Ophthalmology (AAO) Retina Subspecialty Day; October 21, 2011; Orlando, Florida.
Abstract 21.
12. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions:
long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119:802–809.
13. Googe J, Brucker AJ, Bressler NM, et al. Randomized trial evaluating short-term effects of intravitreal
ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular
edema in eyes also receiving panretinal photocoagulation. Diabetic Retinopathy Clinical Research Network.
Retina. 2011;31:1009–1027.
14. Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred
laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–614.
15. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal
bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data:
report 3. Arch Ophthalmol. 2012. Epub ahead of print.
16. Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone
acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–635.
17. Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United
States. Arch Ophthalmol. 2004;122:564–572.
18. Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for
neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol. 2010;150:315–324.
19. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for
neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol.
2009;148:43–58.
© 2012 Direct One Communications, Inc. All rights reserved.
44
References
20. Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in
neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–671.
21. Schmidt-Erfurth U, Eldem B, Guymer R, et al. Efficacy and safety of monthly versus quarterly ranibizumab
treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology.
2011;118:831–839.
22. Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal
neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–1183.
23. Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor trap-eye for macular edema
secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study.
Ophthalmology. 2012;119:1024–1032.
24. Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the
safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology.
2009;116:1731–1739.
© 2012 Direct One Communications, Inc. All rights reserved.
45