Supplemental Content
advertisement

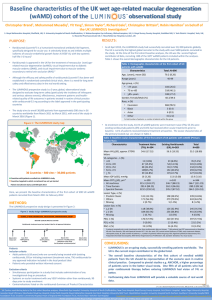
JAMA Ophthalmology Journal Club Slides: Visual Function After Ranibizumab Mitchell P, Bressler N, Tolley K, et al; RESTORE Study Group. Patient-reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmol. Published online August 22, 2013. doi:10.1001/jamaophthalmol.2013.4592. Copyright restrictions may apply Introduction • Importance: – Few data are available on relative changes in vision-related function after treatment for diabetic macular edema (DME). – DME is a major cause of visual impairment and can negatively affect patientreported visual function. – The Ranibizumab Monotherapy or Combined With Laser vs Laser Monotherapy for Diabetic Macular Edema (RESTORE) trial, a phase 3, randomized, doublemasked, 12-month study at outpatient retina practices in Australia, Canada, and Europe, showed that ranibizumab therapy (with or without combined laser) was superior to laser alone for improving best-corrected visual acuity in patients with visual impairment due to DME. • Objective: – To determine the impact of intravitreal ranibizumab, 0.5 mg, compared with laser alone on patient-reported visual function using the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) in the RESTORE trial. Copyright restrictions may apply Methods • Study participants were assigned randomly to ranibizumab plus sham laser (n = 116), ranibizumab plus laser (n = 118), or sham injections plus laser (n = 111). • Ranibizumab and sham injections were given for 3 consecutive months then as needed based on visual acuity; laser and sham laser treatments were given at baseline then as needed. • The NEI VFQ-25 was administered at baseline and months 3 and 12. • The analysis population included all patients receiving 1 or more study treatments with at least 1 postbaseline NEI VFQ-25 assessment. • Last observation carried forward (LOCF) was used for missing postbaseline data. Copyright restrictions may apply Results VFQ-25 Composite Score: Overall Mean Change From Baseline (All 25 Questions) Copyright restrictions may apply Results VFQ-25 General Vision Score: Mean Change From Baseline Copyright restrictions may apply Results VFQ-25 Near Activities Score: Mean Change From Baseline Copyright restrictions may apply Results VFQ-25 Distance Activities Score: Mean Change From Baseline Copyright restrictions may apply Results VFQ-25 Composite Score: Subgroup Analysis by Baseline Visual Acuity Copyright restrictions may apply Results VFQ-25 Composite Score: Subgroup Analysis by Baseline Central Retinal Thickness Copyright restrictions may apply Comment • Vision-related, patient-reported outcomes mirror visual acuity outcomes and support superiority of ranibizumab or ranibizumab plus laser treatment over laser monotherapy for patients with DME and characteristics similar to those enrolled in the RESTORE randomized clinical trial. • Subscale analyses suggest that improvements in the NEI VFQ-25 score are most pronounced for the near activities and general vision subscales. Copyright restrictions may apply Contact Information • If you have questions, please contact the corresponding author: – Paul Mitchell, MD, PhD, Department of Ophthalmology, The University of Sydney, Eye Clinic, Westmead Hospital, Hawkesbury Road, Westmead, New South Wales 2145, Australia (paul.mitchell@sydney.edu.au). Conflict of Interest Disclosures • Dr Mitchell reported receiving consultancy fees, lecture fees, and travel support from Novartis Pharma AG, Pfizer, Solvay (Abbott), Bayer, Alcon, and Allergan. Novartis Pharma AG also funds a retina fellowship at Westmead Hospital, Sydney, which Dr Mitchell reported serving as supervisor. Dr Bressler reported serving as principal investigator of grants at The Johns Hopkins University sponsored by the following entities (not including the National Institutes of Health): Bayer, Genentech Inc, Lumenis Inc, National Eye Institute, Notal Vision, Novartis Pharma AG, Optovue, Regeneron Pharmaceuticals Inc, and The EMMES Corporation. Messers Tolley and Wood reported receiving consultant fees by Novartis Pharma AG to analyze these data. Ms Gallagher reports being a former employee of Novartis Pharma AG, Basel, Switzerland, and currently employed by Sanofi, Cambridge, Massachusetts. Drs Petrillo and Ferreira report being employees of Novartis Pharma AG. Dr Bandello reports serving as an advisory board member for Alcon, Inc, Alimera Sciences, Inc, Allergan, Inc, Bausch and Lomb, Bayer Schering Pharma, Farmila-Thea, Genentech, Inc, Hoffmann-LaRoche, Ltd, Pfizer Inc, Novartis Pharmaceuticals Corporation, sanofi-aventis, and Thrombogenics, Inc. Copyright restrictions may apply