The Diabetic Retinopathy Clinical Research Network Protocol T
advertisement

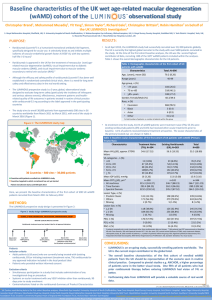
The Diabetic Retinopathy Clinical Research Network Protocol T Comparative Effectiveness Study of Aflibercept, Bevacizumab and Ranibizumab for DME 1 Background Ranibizumab is safe, efficacious, and gives superior visual acuity results to laser for treating eyes with DME (e.g., Phase 3 trials of DRCR.net, RESTORE, RISE & RIDE) However: • High cost can be a barrier to ranibizumab’s widespread use (~$2,000/dose) in the world • Bevacizumab is much less expensive but unknown if it is superior, equivalent, or inferior to ranibizumab • Also reasonable to investigate alternative anti-VEGF agents that are currently available Alternative Anti-VEGF Drugs to Ranibizumab for DME Bevacizumab is a good candidate for direct comparison • • • • • Closest molecular structure to ranibizumab Costs less (~$50 to $100) Widely available Evidence suggests efficacy compared with laser (BOLT 2011) Already widely used for DME Alternative Anti-VEGF Drugs to Ranibizumab for DME Aflibercept • • Approved by FDA for treatment of AMD Phase 2 DME results: o o • • Baseline mean VA: 20/63 in aflibercept arms and 20/80 in laser arm Eyes treated with aflibercept had greater mean improvement in VA at week 52 compared with eyes treated with macular laser (+9.7 to +13.1 letters gained versus -1.3 letters) – DA VINCI, 2012 Unit dose similar to ranibizumab Potential to decrease treatment burden: q4 wk x3 followed by q8 wk equivalent to q4 wk ranibizumab for CNV in AMD (VIEW 1 and VIEW 2) – Note: in CATT, ranibizumab PRN with q4 week OCT equivalent to q4 wk ranibizumab Study Objective and Treatment Arms To compare the efficacy and safety of (1) intravitreal aflibercept, (2) intravitreal bevacizumab, and (3) intravitreal ranibizumab when given to treat central-involved DME in eyes with visual acuity of 20/32 to 20/320. 2.0 mg intravitreal aflibercept 1.25 mg intravitreal bevacizumab 0.3 mg intravitreal ranibizumab Sample Size 660 eyes Participants can have only one study eye If both eyes are eligible, and the patient and the investigator are willing to have either eye as the study eye: • • The eye that never received anti-VEGF treatment should be randomized If both eyes have previously received anti-VEGF treatment or both eyes have never received antiVEGF treatment, then the study eye should be selected by the investigator and the participant Major Inclusion Criteria Age ≥18 years Type 1 or 2 diabetes Study eye: • Visual acuity (~Snellen equivalent) 20/32 or worse and 20/320 or better • Definite retinal thickening due to central-involved DME on clinical exam • OCT central subfield ≥ OCT-machine gender specific cut-off for definite central involved DME Non-study eye: • Investigator must be willing to use (or switch to using) randomized anti-VEGF drug on the non-study eye if needed Major Exclusion Criteria Systemic • Significant renal disease • BP > 180/110 o Based on average of 3 measurements using DRCR.net provided BP device MI, other acute cardiac event requiring hospitalization, stroke, TIA, or treatment for acute CHF within 4 months Study eye • History of intravitreal anti-VEGF within past 12 months • o • • • Enrollment limited to a maximum of 25% of the planned sample size with any history of anti-VEGF treatment History of DME treatment other than anti-VEGF (e.g., macular laser) within past 4 months Macular edema not due to DME PRP in last 4 months or anticipated in next 6 months Baseline Testing E-ETDRS VA testing in each eye OCT on the study eye (spectral domain preferred if available) Ocular exam on each eye Fundus photos in the study eye Blood pressure measurement • Using study provided device Laboratory testing: • • • Urine sample – sent to University of Minnesota HbA1c – sent to University of Minnesota Plasma sample (ancillary study) – sent to Fred Hutchinson Cancer Research Center Follow-up Schedule Baseline to 1 Year • Visits every 4 weeks • Primary outcome at 1 year Optional Visit 23 Days after 1st, 2nd or 3rd visit • Urine sample • Blood pressure (another BP measurement will be taken at the first 4 week visit after the optional visit) 1 Year to 2 Years • Visits every 4 to 16 weeks • Depends on disease status and treatment Follow-up Testing All Protocol Visits: E-ETDRS VA testing in each eye including protocol refraction in the study eye OCT on the study eye (same device as at baseline) Ocular exam on the study eye at each visit and on the non-study eye only if treatment planned Additional Procedures at 1-year Visit Only: Non-study eye refraction and ocular exam Fundus photos in the study eye Blood pressure measurement (3 measurements taken at the visit) Urine sample collection Plasma collection (also taken at the 4-week visit) Principles of DRCR.net DME Treatment: Intravitreal Anti-VEGF Improving on OCT or VA Inject • Worsening on OCT or VA Inject • Improving = OCT central subfield thickness decreased by ≥ 10% or VA letter score improved by ≥ 5 Worsening = OCT central subfield thickness increased by >10% or visual acuity letter score decreased by >5 Stable: not improving or worsening on OCT or VA Inject unless stable since last 2 injections in which case inject only if before 24wk visit when OCT is >250 µm and VA <20/20 Principles of DRCR.net DME Treatment: Intravitreal Anti-VEGF: When eye is stable after at least 2 consecutive injections and OCT CSF is <250 µm and VA is 20/20 or better Defer injection When eye is stable after at least 2 consecutive injections and OCT CSF is ≥250 µm or VA is worse than 20/20: • • Prior to the 24-week visit Inject ≥ 24-week visit Defer injection Focal/Grid Laser Treatment Criteria Laser can be initiated only at or after the 24 week visit if: • • OCT CSF is ≥250 µm or there is edema that is threatening* the fovea AND The eye has not improved on OCT or VA compared with either of the last two consecutive injections *Edema threatening the fovea is defined as edema within 500 µm of the foveal center or edema associated with lipid within 500 µm of the foveal center or ≥ 1 disc area of edema the posterior edge of which is within 1 disc diameter of the foveal center Focal/Grid Laser Re-Treatment Once focal/grid laser has been initiated, retreatment with focal/grid laser will be given unless one of the following is present: • • • • Focal/grid laser has been given in <13 weeks The OCT CSF is <250 and there is no edema threatening the fovea Complete focal/grid laser has been given already The OCT or VA has improved since the last laser Intravitreal Anti-VEGF Treatment in the Non-study Eye If the non-study eye is treated for any condition which requires treatment with an anti-VEGF agent, the non-study eye must be treated with the same drug to which the study eye was randomized The DRCR.net CC will provide drug for the nonstudy eye For participants that have a study eye randomized to ranibizumab the non-study eye can receive one of the following: o o 0.5mg for treatment of conditions other than DME in the non-study eye 0.3mg for DME treatment in study eye and non-study eye Referrals Please consider any eyes with definite retinal thickening due to central-involved DME Must be willing to be randomized to any of the three treatment groups and continue in the study for 2 years Consenting/Enrollment/Randomization may be split into separate visits 17 Thank You on Behalf of Diabetic Retinopathy Clinical Research Network (DRCR.net) Dedicated to multicenter clinical research of diabetic retinopathy, macular edema and associated disorders. 18