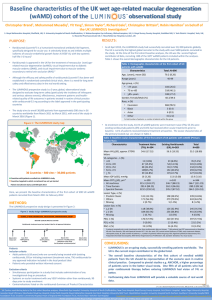
Diabetic Retinopathy Clinical Research Network
advertisement

AAO Media Briefing AMD Trials and Treatments: An Evolving Landscape ABDHISH R. BHAVSAR, M.D. Chair 1 Financial Disclosures Abdhish R. Bhavsar, MD Research support - clinical trials: DRCR, Regeneron, Genentech 2 Agenda Abdhish R. Bhavsar, MD: Welcome/Introductions Pravin Dugel, MD: Fovista 2b clinical trial results • Q/A Abdhish R. Bhavsar, MD: Update CATT and Eylea Susan Bressler, MD: Cataract sugery/AMD - no inc risk Paul Mitchell, MD: Cataract surgery/AMD – inc risk Edwin Stone, MD: Update on genetic testing for AMD Media Q & A 3 Study Disclosures • This study does include research conducted on human subjects • IRB approval has been obtained for each of the studies discussed. V 2.0; Oct 2012 Integrated VIEW 1 Investigation of Efficacy and Safety of Intravitreal Aflibercept Injection in Wet Age-Related Macular Degeneration (AMD) 7 8 Study Design Multi-center, active controlled, double masked trial VIEW 1 N=1217; VIEW 2 N=1240 Patients randomized 1:1:1:1 Intravitreal Ranibizumab Aflibercept Primary endpoint: Maintenance of Vision *After 3 initial monthly doses Dosing through Week 52 Modified quarterly dosing through Week 96 Secondary endpoint: Mean change in BCVA Study Endpoints PRIMARY ENDPOINT • Proportion of patients who maintained BCVA (%) (losing <15 ETDRS letters from baseline) • Mean change in BCVA as measured by ETDRS letter score from baseline KEY SECONDARY ENDPOINTS BCVA: Best-Corrected Visual Acuity • Proportion of patients who gained at least 15 letters of BCVA from baseline • Central Retinal Thickness V 2.0; Oct 2012 Integrated VIEW 1 Results Week 52 16 17 18 20 V 2.0; Oct 2012 Integrated VIEW 1 Results Week 52 – Week 96 Treatment Schedule Re-treatment Criteria – • 12 weeks since previous injection • New or persistent fluid on OCT • Increase in CRT of ≥100 μm compared to the lowest previous value • Loss of ≥5 ETDRS letters from the best previous score in conjunction with recurrent fluid on OCT • New onset classic neovascularization • New or persistent leak on FA • New macular hemorrhage Solid = Injection Outline = Sham Hatched = Modified Quarterly Dosing V 2.0; Oct 2012 Integrated VIEW 1 Safety Summary Aflibercept noninferior to ranibizumab Safety and efficacy was similar amongst the treatment groups 41 Comparison of AMD Treatments Trials (CATT): Two Year Results Abdhish R. Bhavsar, MD for the Comparison of AMD Treatments Trials (CATT) Research Group Supported by Cooperative Agreements from the National Eye Institute, National Institutes of Health, DHHS 46 Objectives To determine the relative efficacy and safety of intravitreal ranibizumab and bevacizumab for treatment of neovascular AMD To determine if less than monthly dosing of either drug compromises long term visual outcomes - Designed as two-year study with primary outcome at one year 47 CATT Clinical Sites 1185 patients with neovascular AMD enrolled at 43 sites in the United States 48 Enrollment Criteria More Inclusive than Previous AMD Trials CNV not required to be subfoveal as long as center involved by some component such as SRF, PED, or blood. Allowed RAP lesions, juxtafoveal, and extrafoveal CNV Allowed eyes with VA 20/25-20/320 No limit on size of lesion 49 Enrollment Criteria More Inclusive than Previous AMD Trials Allowed eyes with >50% blood. All other entry criteria had to be met (VA 20/320 or better and can identify CNV on FA and fluid on OCT) 50 50 CATT Treatment (Months) Year 1 0 1 2 3 4 5 6 7 8 Year 2 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 ranibizumab Monthly bevacizumab Monthly ranibizumab PRN bevacizumab PRN } Retreat if fluid on OCT or other signs of active CNV Primary Endpoint Final visit 51 Treatment in PRN Arms Treat to a dry OCT – zero tolerance for intraretinal, subretinal, or sub-RPE fluid. May also treat if there is other evidence of CNV activity New subretinal or intraretinal hemorrhage Leakage or increased lesion size on FA Unexplained decrease in visual acuity with no obvious atrophy or subretinal fibrosis. No retinal thickness threshold (100 microns) as used in many neovascular AMD treatment studies. 52 CATT Study Drugs Ranibizumab supplied locally similar to patients outside of the study Bevacizumab supplied by CATT repackaged in glass vials under IND Mean Change in Visual Acuity All Groups 54 Mean Change in Total Retinal Thickness Over Time All Groups Percent with No Fluid at 1 Year 100% Lucentis Monthly (n=284) 90% Percentage of Patients 80% Avastin Monthly (n=265) Lucentis PRN (n=285) Avastin PRN (n=271) P < 0.001 70% 60% 50% 43.7% 40% 30% 20% 26.0% 23.9% 19.2% 10% 0% 57 Year 1 Adverse Events No difference between drugs in rates of death, stroke, or myocardial infarction Imbalance in total SAE’s (mostly hospitalizations): 24% bevacizumab vs 19% ranibizumab (p=0.04) SAEs broadly distributed across all organ systems with differences present in areas not previously identified as areas of concern in systemic bevacizumab trials. 58 Questions at End of Year 1 Would ranibizumab and bevacizumab remain equivalent for visual acuity in Year 2? Would wider visual acuity differences emerge between monthly and PRN dosing in Year 2? Would the fluid differences between treatments noted in year 1 impact visual acuity with longer follow-up? Would switching to PRN dosing after one year of monthly treatment maintain or adversely effect vision? Would important safety differences emerge with longer follow-up? 59 Two Year Results 60 Patients 1107 patients alive who continued in Year 2 All available monthly treated patients in Year 1 (n=549) were successfully randomized to monthly or PRN treatment in Year 2 Masking remained robust in Year 2 with identity of assigned drug known to ophthalmologist in only 66 of 12,645 evaluations (11 patients) 62 Visual Acuity Results Same Regimen for Two Years 64 Mean Change in Visual Acuity Same Regimen for Two years Patients Without 15 Letter Decrease Same Regimen for 2 Years 66 Anatomical Results Same Regimen for Two Years 69 PercentSame with No Fluid on OCT Regimen for 2 Years 71 Typical Amount of Residual Fluid 72 PercentSame with Geographic Atrophy Regimen for 2 Years 73 Effect of Switching to PRN after One Year of Monthly Dosing 77 Mean Change in Visual Acuity after Week 52 Monthly Always and Switched to PRN Percent with No Fluid on OCT Monthly Always and Switched to PRN 81 Percent with Geographic Atrophy Monthly Always and Switched to PRN 82 Adverse Events 89 Death and APTC Events ranibizumab bevacizumab (N=599) (N=586) Difference Death 95% CI P 32 (5.3%) 36 (6.1%) 0.8% (-1.9%, 3.5%) 0.62 APTC* 28 (4.7%) 29 (5.0%) 0.3% (-2.2%, 2.8%) 0.89 *Includes nonfatal myocardial infarction, nonfatal stroke, and vascular deaths 90 Cumulative Proportion with a Systemic Serious Adverse Event 91 Any Systemic Serious Adverse Event Drug Difference Unadjusted 2-year rates ranibizumab 190 /599 (31.7%) bevacizumab 234 /586 (39.9%) Adjusted Risk Ratio 8.2% 1.30 95% CI P (2.8%, 13.6%) 0.004 (1.07, 1.57) 0.009 Regimen Unadjusted 2-year rates* Monthly 199 /587 (33.9%) PRN 225 /598 (37.6%) Adjusted Risk Ratio § 3.7% 1.20 *Regimen as originally assigned §PRN a time dependent covariate in Cox model (-1.7%, 9.1%) 0.18 (0.98, 1.47) 0.08 92 Systemic Serious Adverse Events ranibizumab (N=599) bevacizumab (N=586) Difference (95% CI) P Associated w/ Anti-VEGF* Yes 45 ( 7.5%) 62 ( 10.6%) 3.1% (-0.2%, 6.4%) 0.07 No 170 (28.4%) 202 (34.5%) 6.1% (0.8%,11.3%) 0.02 * Arteriothrombotic events (myocardial infarction, stroke), systemic hemorrhage, congestive heart failure, venous thrombotic events, hypertension, vascular death. 93 Ocular Adverse Events Endophthalmitis – 0.06% (11 /18,509 injections) - 11 cases (4 ranibizumab, 7 bevacizumab) - 10 of 11 cases in monthly treatment group; PRN case had received 22 injections Pseudo-endophthalmitis ranibizumab 1 bevacizumab - 1 Ocular HTN or Glaucoma ranibizumab - 15 bevacizumab - 14 94 Dollars 2-Year Drug Cost Per Patient 50,000 45,000 40,000 35,000 30,000 25,000 20,000 15,000 10,000 5,000 0 Lucentis Monthly Avastin Monthly Lucentis PRN Avastin PRN 95 Summary Ranibizumab and bevacizumab were equivalent for visual acuity at all time points over a 2-year period. PRN treatment resulted in less gain in visual acuity (-2.4 letters) at 2 years but vision for all groups was similar at end of 2 years. PRN dosing resulted in mean of 10 fewer injections over 2 years than monthly dosing. Bevacizumab patients received mean 1.5 more injections than ranibizumab. More eyes were completely dry on OCT with monthly dosing with the highest rate in eyes receiving ranibizumab monthly. More eyes developed geographic atrophy with monthly dosing with the highest rate in eyes receiving ranibizumab monthly. 97 Summary PRN groups had more leakage on FA and more lesion growth than monthly groups. Switching to PRN after one year of monthly treatment produced visual and anatomical results that were similar to PRN-always. There were no differences between drugs in rates of death or arteriothrombotic events. Bevacizumab treated patients had higher rates of systemic SAEs than ranibizumab treated patients. The reason for this difference remains unclear given the lack of specificity to conditions associated with inhibition of VEGF. 98 Future Improved drug delivery systems Treatments for non-exudative AMD Targeted treatments with combinations of drugs Better prevention strategies 110 Additional Questions Mary Wade, Academy Science and International PR Manager mwade@aao.org Cell: 510-725-5677 111 Thank You! 112