Parkinson Disease - GRECC Audio Conferences
advertisement

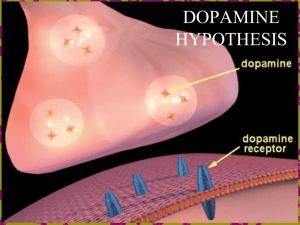
Parkinson Disease: Beyond Dopamine Roger L. Albin, M.D. Ann Arbor VAHS GRECC & Dept. of Neurology, University of Michigan Epidemiology of PD • Estimated prevalence = 300-350/100,000 or 840,000 – 1,000,000 in the USA; likely an underestimate • Estimated incidence = 10-15/100,000 • Exponential increase with age over 60 • Risk modifiers – Occupation? – Heavy Metal Exposure? – Smoking is Protective? • Genetic Component? (Twins vs Icelanders) Parkinsonism: A Dopamine Deficiency Syndrome • • • • Slow voluntary movement - Bradykinesia “Stiff” muscles - Rigidity Falling - Postural Instability Shaking - Resting Tremor All above features seen in dopamine depletions, dopamine receptor antagonist exposures and in degenerations of the nigrostriatal dopaminergic projections Differential Diagnosis of Parkinsonism • Pharmacologically Induced – Dopamine Antagonists; Anti-Psychotics, Anti-Emetics – Catecholamine Depleters; Reserpine, Tetrabenazine – False Transmitters; a-Methyl-Tyrosine • Essential Tremor - Not Really a Mimic • Atherosclerotic Parkinsonism • Other Neurodegenerations Affecting the Basal Ganglia – Progressive Supranuclear Palsy – Multiple Systems Atrophy – Corticobasal Degeneration Syndrome • Idiopathic Parkinson’s Disease United Kingdom Parkinson’s Disease Brain Bank Diagnostic Criteria Step 1: Diagnosis of Parkinsonism • Bradykinesia and at least one of the following: Muscular rigidity 4–6 Hz resting tremor postural instability not caused by primary visual, vestibular, cerebellar or proprioceptive dysfunction Step 2: Features tending to exclude Parkinson’s disease as the cause of Parkinsonism History of repeated strokes with stepwise progression of parkinsonian features History of repeated head injury History of definite encephalitis Neuroleptic treatment at onset of symptoms >1 affected relatives Sustained remission Strictly unilateral features after 3 years Supranuclear gaze palsy Cerebellar signs Early severe autonomic involvement Early severe dementia with disturbances of memory, language and praxis Babinski's sign Presence of a cerebral tumour or communicating hydrocephalus on computed tomography scan Negative response to large doses of levodopa (if malabsorption excluded) MPTP exposure Step 3: Features that support a diagnosis of Parkinson’s disease (three or more required for diagnosis of definite Parkinson’s disease) Unilateral onset Rest tremor present Progressive disorder Persistent asymmetry affecting the side of onset most Excellent (70–100%) response to levodopa Severe levodopa-induced chorea Levodopa response for ≥ 5 years Clinical course of ≥ 10 years How Common and What are They? • 90% of Parkinsonism is PD – About 3-4% is PSP – About 3-4% is MSA – A Grab Bag For the Rest • Queen Square Autopsy Series (70 Cases) – 35 MSA – 20 PSP – 4 Unknown – 3 CBD – 3 Vascular Parkinsonism – 2 Post-Encephalitic Parkinsonism Diagnostic Accuracy For PD • Historically Not Very Good – 75%. • Greater Awareness of PD Mimics Has Improved Accuracy • UK PD Brain Bank Study – 90% for PD – Involved general neurologists, subspecialty neurologists, geriatric specialists, GPs • Queen Square Study of Movement Disorder Specialists – PPV of 98.6% Twin Concordance Rates Relative Type No. of Relatives No. Affected (%) RR (95% CI) p Relatives of PD 2,865 71 (2.5) 2.7 (1.74.4) <0.00 01 Relatives of controls 2,446 25 (1.0) 1.0 (reference) Relatives of early-onset PD 1,172 27 (2.3) 2.9 (1.65.0) 0.000 2 Relatives of late-onset PD 1,693 44 (2.6) 2.7 (1.64.4) 0.000 2 Relatives of controls 2,446 25 (1.0) 1.0 (reference) Siblings of early-onset PD 482 8 (1.7) 7.9 (2.525.5) 0.000 5 Siblings of late-onset PD 587 14 (2.4) 3.6 (1.310.3) 0.02 Siblings of controls 889 5 (0.6) 1.0 (reference) Parents of early-onset PD 426 17 (4.0) 1.7 (0.93.3) 0.2 Parents of late-onset PD 505 29 (5.7) 2.5 (1.44.6) 0.003 Parents of controls 789 19 (2.4) 1.0 (reference) Relatives of tremordominant PD 989 27 (2.7) 2.6 (1.44.6) 0.002 Relatives of PIGD PD 1,460 36 (2.5) 2.9 (1.75.0) <0.00 01 Relatives of controls 2,446 25 (1.0) 1 (reference) PD Risk in Icelanders Park1 (aSynuclein) 4q421 AD Late Onset Lewy Bodies Park2 (Parkin) 6q25 AR (AD) Early Onset No Lewy Bodies Park5 (UCHL1) 4p14 AD Late Onset ? Park3 2p13 AD Late Onset Lewy Bodies Park4 4p14-16.3 AD Late Onset Lewy Bodies Park6 (PINK1) 1p35-36 AR Late Onset ? Park7 (DJ-1) 1p36 AR Early Onset ? Park8 (LRRK2) 12p11.2-q13 AD Late Onset Variable Lewy Bodies Park9 (ATP13A2) 1p36 AR Early Onset ? Park 10 1p32 ? Late Onset ? NR4A2 (NURR1) 2a22-23 AD Late Onset ? Park1 - aSynuclein • First Locus Identified • Widely Expressed Synaptic Protein – Normal Function Unknown • Primary Constituent of Lewy Bodies • Contursi Kindred and Other Pedigrees – Many Typical Clinical Features with Somewhat Earlier Age of Onset • A53T Mutation • A30P Mutation – German Pedigree • E46K Mutation – Spanish Pedigree; Lewy Body Dementia • Iowa (Spellman-Muenter) Kindred – Triplication • Recent Description of Duplication Pedigrees Park8 – LRRK2 • Function Unknown – GTP binding and Kinase domains • Relatively Common – 1-2% of apparently sporadic PD in some studies • Founder Effects • Incomplete Penetrance • Royal Road to Mechanisms of Pathogenesis? Fig. 2. A model of mitochondria and PD pathogenesis Li, Chenjian and Beal, M. Flint (2005) Proc. Natl. Acad. Sci. USA 102, 16535-16536 Copyright ©2005 by the National Academy of Sciences Initial Treatment Options • No Treatment - No Functional Disability • Anti-Cholinergics - Tremor • Amantadine - Mild Disability • L-Dopa/Carbidopa • Dopamine Agonists • Selegiline Dopaminergic Synapse L-DOPA AADC Dopamine VMAT2 D2 Receptor DAT PD Therapy: L-DOPA • Advantages: Provides “natural”/”regulated” effect in early PD. • Mechanisms: Increased quantal size (low-dose, mild PD), increased dopamine release, reduced clearance. • Preparations: Regular vs. Continuous Release. • Disadvantages: Dietary Interactions, complex pharmacokinetics and pharmacodynamics. Parkinson’s Disease: Natural History in the Post-L-DOPA Era Dopamine/L-Dopa Toxicity? ELLDOPA Basic Principles of Using LDopa • Give enough Carbidopa - Start with 25/100 tid with meals. • Use immediate release initially. • “Enough is as good as a feast” – titrate to clinical effect. • Almost no drug interactions. • Almost no medical contraindications. • Provide adequate time to assess response. Adapted from Obeso, et al, 1997 Clinical Effect Dyskinesia Threshold Response Threshold Time (Hrs) Time (Hrs) Time (Hrs) •Early PD Moderate PD Advanced PD •Long-duration response •Short-duration response •Short-duration response •Low incidence of dyskinesia •Increased dyskinesias •Narrow window Year 0-5 Year 610 Year >11 PD Therapy: Dopamine Agonists • Advantages: Sustained, steady-state plasma levels; No dietary effects on CNS availability; Convenient dosing regimens • Mechanism: Stimulation of D2-type receptors • Disadvantages: Unregulated effect on all D2 receptors; More frequent side-effects • Use non-ergot agents because of fibrotic complications. • Speculative long term benefits. CALM-PD Design • Subjects – Early PD requiring Dopaminergic Treatment – No L-dopa or pramipexole x 2 months – No motor complications • Intervention – Ascending Pramipexole; 4.5 mg/day max – Ascending L-dopa; 150-600/day max – Open label additional L-dopa allowed Symptomatic vs Protective Effects Impulse Control Disorders • Pathologic Gambling, Sexual Behavior, Compulsive Shopping • Strongly Associated with Dopamine Agonists • Not Uncommon: 4% - 8% in some good clinic series • Rare with L-dopa Monotherapy • Reversible CALM-PD Summary • No Evidence for Neuroprotection • Effect on Wearing Off? – Use of Long Acting Agents – Inappropriate Design • Effect on Dyskinesias? – Lack of Therapeutic Equivalence • Generalizability of Trial – Age of Subjects – Health of Subjects – Cognitive Status The Bottom Line • Dopamine agonists and L-Dopa are both reasonable choices for initial therapy. • L-Dopa possesses advantages for symptomatic treatment. • The long-term benefits of dopamine agonists are hypothetical. What to Do? • Experts Differ Somewhat. • <60 years – Tendency to use agonist initial therapy • 60 – 65 and healthy – Consider agonist initial therapy • > 65 years and anyone with hint of cognitive impairment, other medical problems, or complex medical regimens – L-dopa • Financial Issues – L-dopa Important Later Clinical Features • Major Contributors to Disability • Refractory Speech and Swallowing Problems • Marked Postural Instability - Falls • Refractory Gait Problems • Autonomic Insufficiency • Dementia: 30% - 50% • Hallucinations and Psychosis • Sleep Disorders • Constipation Hallucinations Very Common Characteristic Features Not necessarily psychosis Insight preserved often Often medication related If infrequent or not bothersome; reassure If treatment needed – quetiapine preferred Harbinger of dementia Dementia • Very Common: 40% - 50% in some estimates. 70% in one recent community survey. • Lewy Body Type Features – Parkinsonism – Hallucinations – Fluctuations • Benefit with Cholinesterase Inhibitors? • Bad Prognostic Predictor Sleep Disorders • Sleep Disruption and Daytime Somnolence Very Common • Many Problems with Sleep • REM Sleep Behavior Disorder – History from sleep partners – May herald PD • Obstructive Sleep Apnea Contact Information Roger L. Albin, MD Rm 202, Bldg 31 Ann Arbor VA 2215 Fuller Road Ann Arbor, MI, 48105-2399 734-845-5466 ralbin@umich.edu