Gout - American Osteopathic Association
advertisement

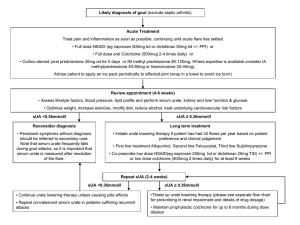
Treatment Update For Gout The Future is Now Scott R. Burg, D.O. Orthopaedic and Rheumatologic Institute Cleveland Clinic Gout…in 1799… Flare: Classic Description The victim goes to bed and sleeps in good health. About two o’clock in the morning he is awakened by a severe pain in the great toe; more rarely in the heel, ankle, or instep. The pain is like that of a dislocation, and yet the parts feel as if cold water were poured over them . . . Now it is a violent stretching and tearing of the ligaments – now it is a gnawing pain, and now a pressure of tightening. So exquisite and lively meanwhile is the feeling of the part affected, that it cannot bear the weight of the bedclothes nor the jar of a person walking in the room. The night is spent in torture. Sydenham, 1683 Sydenham, T: The Works of Thomas Sydenham, London, New Sydenham Soc. 1850 (translation) Gout Defined As… The deposition of monosodium urate crystals (MSU) in tissues at physiologic pH What is Gout? • Gout is a disorder of uric acid metabolism • This is NOT just a disease of the joints! • Characterized by: - Hyperuricemia - Attacks of acute arthritis - Tophi around joints - Joint destruction - Renal Disease (Glomerular, Interstitial, Tubular) Dz - Uric Acid Urolithiasis Untreated Gout May Lead to… • Tophaceous masses of MSU crystals in cartilage and joints • Renal stones • Urate nephropathy Peripheral Structure Involvement vs Relative Sparing of Central Joints • Attacks due in part to temperatures <37 C peripherally • Resulting in reduced solubility of urate Total Population (Millions) Total Population of Several Rheumatic Conditions in the United States 9 8.3 Million* 8 7 7.1 † Million 5.0 ‡ Million 6 5 2.7 § Million 4 3 2 1.3 ‡ Million 1 0 Gout Activity-limiting Back Pain Fibromyalgia *Based on patient-reported data from NHANES 2007-2008. †Estimated from 1997 NHIS and 2005 US Census Bureau data. ‡Estimated from 2005 US Census Bureau data. §Based on 1988 NHIS data. Carpal Tunnel Syndrome Rheumatoid Arthritis Who May Be the Patient With Hyperuricemia and Gout? Comorbidities Demographics • Advanced age • Male • Postmenopausal women • • • • • • Lifestyle Commonly Used Medications • • • • Diuretics Low-dose aspirin (eg, <325 mg) Cyclosporine Niacin Hypertension Cardiovascular disease Chronic kidney disease Diabetes mellitus Dyslipidemia Metabolic syndrome • • • • Obesity (high BMI) Diet rich in meat and seafood High alcohol intake Frequent consumption of high-fructose corn syrup Risk Factors for Gout (Continued) Generic Overproducers Some patients have unusual shunt mechanism that converts glycine directly to uric acid HPRT deficiency Lesch-Nyhan Syndrome Gout and Mental Retardation in Children X-linked PRPP hyperactivity G6PD deficiency Autosomal recessive Von Gierke’s type-1 glycogen storage disease Fructose Intake and Urate Excretion HFCS Pyruvate Purine Catabolism Fructose AMP Lactate Uric Acid ATP Fructose 1Phosphate • Dominant dietary source – high-fructose corn syrup (HFCS) • High concentration of fructose causes rapid accumulation of AMP - Increases the body pool of purines • Lactic acid is a by-product of fructose metabolism - Lactate decreases urate excretion Diagnosing Gout • Abrupt onset of severe pain, swelling, and tenderness that reaches its maximum within just 6–12 hours, especially with overlying erythema, is suggestive of crystal inflammation though not specific for gout • A presumptive diagnosis is reasonably accurate for typical presentations, such as recurrent podagra with hyperuricemia • Demonstration of MSU crystals in synovial fluid or tophus provides definitive diagnosis Acute Flares • Flares occur without warning and may: - Produce extreme pain - Last hours to weeks - Limit mobility • Monoarticular in ~90% of initial presentations; ~50% are podagra • Over time, flares may occur more often • Temporary reduction in sUA levels can occur during a gout flare, making sUA measurements during a flare unreliable Courtesy of Theodore Fields, MD. Gout Radiograph http://www.pathguy.com Intervals Between 1st & 2nd Acute Flares Majority experience second acute flare within 1 year of first gout flare 1-2 yrs 16% 2-3 yrs- 6% 3-5 yrs - 5% Within 1 yr 62% After 10 yrs - 4% No 2nd in more than 10 yrs - 7% Yu et al. Ann Int Med. 1961;55:179-192 Recommendations From the 2012 American College of Rheumatology Guidelines for Management of Gout • ACR recommends a comprehensive treatment plan for the management of gout, including both nonpharmacologic and pharmacologic approaches1,2 • Patient education including diet and lifestyle modifications is recommended along with the following pharmacologic approaches for the management of gout1,2 Acute Gout Flares • Treat an acute gout flare with pharmacologic therapy (NSAIDs, corticosteroids, or colchicine) within 24 hours of onset2 Gout Flare Prophylaxis • For gout attack prophylaxis, initiate low-dose colchicine or lowdose NSAIDs when initiating uratelowering therapy (ULT)2 • Anti-inflammatory prophylaxis should be continued from initiation of ULT for the greater of2: • At least 6 months, or • Following achievement of target serum urate, for 3 months in patients without or 6 months in patients with tophi on physical exam Chronic Gout Management • When initiating ULT, begin anti-inflammatory gout flare prophylaxis1 • Initiate first-line ULT, febuxostat or allopurinol, or if at least one of these is contraindicated or not tolerated, probenecid can be used to treat to sUA target of <6 mg/dL1 • sUA should be monitored regularly (every 2-5 weeks) during ULT titration, then every 6 months once target sUA is achieved1 Management of Gout RESOLVE INITIATE MAINTAIN Acute Flare Urate-lowering Therapy Treatment to Control sUA Treat with antiinflammatory agents Target sUA <6 mg/dL with ULT Initiate concomitant anti-inflammatory prophylaxis for prevention of mobilization flares Continue ULT to maintain sUA levels and reduce the risk of future flares Continuing prophylaxis for 6 months reduced the frequency of gout flares in a clinical study Track sUA levels NSAIDS • Indomethacin (Indocin) has usually been used • Lots of GI Toxicity / Renal Toxicity • 25mg TID • May use other NSAIDS • Don’t use aspirin, competes with uric acid for excretion in the kidneys Evidence for the Use of Concomitant Anti-Inflammatory Prophylaxis • Anti-inflammatories are used for both acute flares and prophylaxis • ACR guidelines, as well as medical consensus and clinical evidence, support the use of antiinflammatory prophylaxis when initiating ULT • In a clinical study, using colchicine with ULT for 6 months decreased the frequency of gout flares 2.0 Mean Number of Flares1 • Anti-inflammatory prophylactic therapy reduces the risk of mobilization flares, but is not a chronic treatment * Colchicine 0.6 mg twice daily (n=21) Placebo (n=22) 1.5 † 1.0 0.5 0.0 0–3 3–6 Time Interval, Months *P=0.022; †P=0.033. Corticosteroids • Indications for Steroids in the Management of Acute Gouty Arthritis • Co morbid medical illnesses - CHF, HTN Renal Insufficiency Peptic Ulcer, GI Bleed Hepatic Insufficiency Chronic Alcoholism Bleeding diathesis Advanced Age Anticoagulant use Post Op NSAID Hypersensitivity Severe attacks refractory to NSAIDs/Colchicine Corticosteroids • Methods - Parenteral - Oral - Intra-articular • Prednisone - 1mg/kg PO as a single dose -OR- 20-40 mg PO QD taper by 5-10mg every 3 days until D/C • Methylprednisolone - 40 mg intra-articular single dose • Triamcinolone - 40 mg intra-articular as a single dose Colchicine • Alkaloid obtained from autumn crocus • Minimal effect on uric acid synthesis and excretion • Prevents release of chemotactic factors and cytokines from neutrophils • Binds to microtubules in neutrophils • Major use is in acute gouty attacks • 0.6mg - two initially, then one every 2 hours until pain is relieved, you have reached 6mg or diarrhea, nausea or vomiting develop • IV Colchicine no longer available • NSAIDS have largely replaced colchicine Colchicine, USP Overview • Colchicine is approved for 2 gout indications: - Treatment of gout flares - Prophylaxis of gout flares • Colchicine is not an analgesic medication and should not be used to treat pain from other causes Colchicine, USP Dosing Considerations Usage Renal or hepatic impairment ClCr ≥30 mL/min Patients receiving dialysis Severe impairment Dosing No dose adjustment Reduce dose Reduce dose Coadministration with CYP3A4 (eg, clarithromycin, ritonavir) or P-gp inhibitors (eg, cyclosporine) Reduce colchicine dose Renal or hepatic impairment AND Concurrent P-gp inhibitors or strong inhibitors of CYP3A4 Contraindicated, as life-threatening or fatal toxicity has been reported with colchicine in this setting Colchicine Pharmacokinetics in AGREE Trial Concentration (ng/mL) 10 High-dose colchicine (4.8 mg total) Low-dose colchicine (1.8 mg total) Single-dose colchicine (0.6 mg total) 8 6 4 2 0 0 2 4 6 8 10 12 Hours 14 16 18 20 22 24 Colchicine, USP Dosing and Administration: Acute Flares Usage Treatment of gout flares: 1.2 mg at first sign of flare, then 0.6 mg 1 hour later. Maximum dose 1.8 mg over a 1-hour period; higher doses have not been found to be more effective Dosing Colchicine, USP Dosing and Administration: Prophylaxis Usage Prophylaxis of gout flares: For the prevention of mobilization flares, 0.6 mg once or twice daily; maximum dose 1.2 mg/day Dosing Colchicine, USP Effectively Reduced the Pain of Acute Gout Flares Percentage of responders Percentage of responders based on target joint pain score at 24 hours post first dose 40 30 38%* † 33% 20 16% 10 0 Low-dose Colchicine, USP (n=74) High-dose colchicine (n=52) * P=0.005 vs placebo; † P=0.034 vs placebo. Placebo (n=58) • The primary endpoint was the proportion of patients who experienced at least 50% reduction in pain scores from baseline at 24 hours, without rescue medication Colchicine, USP Had a Lower Incidence of Gastrointestinal Adverse Events Treatment-emergent adverse events (AEs) occurring in ≥2% of patients in any treatment group Percentage of patients 100 80 77 77 † High-dose colchicine (n=52) * (n=74) Low-dose COLCRYS Placebo (n=59) 77 60 40 37 27 26 20 20 23 14 17 19 17 4 5 8 0 0 1 2 0 0 0 Any AE Any gastrointestinal disorder Diarrhea Nausea Vomiting General disorders Severe diarrhea and administrationsite conditions Colchicine, USP Dose Adjustments for Coadministration With CYP3A4 Inhibitors Colchicine, USP Dose Adjustments for Coadministration With P-gp or Protease Inhibitors Principal Potential Adverse Events with Colchicine Used to Treat Acute Gout Common with Excess Oral Colchicine • GI - Diarrhea (sometimes severe) Nausea Vomiting Abdominal cramps Dehydration Most common with Overdose of Oral Colchicine • Bone marrow depression: nadir at 7 days • Neuropathy-myopathy, elevated CK, and weakness: onset can be in weeks Less Common with Severe Overdose of Oral Colchicine • CV - • • Cardiac toxicity Arrhythmia Vascular collapse Hepatotoxicity Alopecia Management of Gout RESOLVE INITIATE MAINTAIN Acute Flare Urate-lowering Therapy Treatment to Control sUA Treat with antiinflammatory agents Target sUA <6 mg/dL with ULT Initiate concomitant anti-inflammatory prophylaxis for prevention of mobilization flares Continue ULT to maintain sUA levels and reduce the risk of future flares Continuing prophylaxis for 6 months reduced the frequency of gout flares in a clinical study Track sUA levels Gout Treatment • Acute - Treat the Pain! - NSAIDS • Indocin, Ibuprofen, Naproxen • NOT Asprin! - COX-2 - Colchicine - Corticosteroids • Chronic/Ongoing - Decreased production • Probenecid • Sulfinpyrazone • Increase excretion • Allopurinol • Febuxostat - Do not use these drugs during acute attack since these therapies may initially exacerbate the condition FDA-Approved Urate-Lowering Agents Drug Action First-Line (Uricostatic) Allopurinol Xanthine Oxidase inhibitor Febuxostat Xanthine Oxidase inhibitor Second-Line (Uricostatic) Probenecid URAT1 and GLUT9 inhibitor Dose Range 100-800 mg daily (decrease dose in renal impairment) 40-80 mg daily 500-2000 mg daily (carefully adjust dose to 3000 mg maximum) For Severe, Treatment-Refractory Disease (Uricostatic) Pregloticase IV Recombinant, 8 mg IV every 2 weeks PEGylated uricase Allopurinol • Historically the drug of choice in treatment of chronic tophaceous gout • Competitive inhibitor of xanthine oxidase • Xanthine and hypoxanthine are more soluble and better excreted renally • Metabolized to oxypurinol • Oxypurinol accumulates—may be responsible for antigout effects • Oxypurinol is not well absorbed orally • Decreases serum and urinary uric acid levels Serum Uric Acid Level (mg/dL) Correlation Between Allopurinol Dose and Serum Urate 14 12 10 8 6 4 2 0 0 2 4 6 8 Dose (mg/kg/day) Takada M, et al. J Clin Pharm Ther. 2005;30:407-412 Velocity of Tophus Size Reduction Accelerates as Serum Urate Drops Below 4 mg/dL Serum Urate (mg/dL) 8 6 4 Allopurinol Benzbromarone Combined 2 0 0.0 0.5 1.0 1.5 2.0 2.5 Velocity of reduction in Size of Index Tophus (mm/mo) Perez-Ruiz F, et al. Arthritis Rheum. 2002;47)4):356-360 Gout Subject Achieving SUA <6.0 mg/dL (%) Dose Adjustment of Allopurinol According to Creatinine Clearance Does Not Provide Adequate Control of Hyperuricemia in Patients With Gout 100 80 P <0.01 60 38 40 20 19.1 15 0 Lower than recommended Recommended Higher than recommended Dalbeth N, et al. J Rheumatol. 2006;33:1646-1650 Drugs Potentially Affected by Allopurinol Therapy • Ampicillin/amoxicillin (~20% risk of ampicillin/amoxicillinrelated rash) • Azathiprine* • Chlorpropamide • Cyclophosphamide • Dilantin • • • • • • • Dilantin 6-Mercaptopurine* Probenecid Theophylline* Vidarabine Warfarin ACE inhibitors (suspected) *Potential for severe toxicity by impairment of drug clearance via suppression of xanthine oxidase. Potential for drug-drug interaction is also highly significant with the xanthine oxidase inhibitor febuxostat. Becker M, et al. Arthritis and Allied Conditions, 14th ed. Philadelphia, PA. Lippincott, Williams and Wilkins’ 2001:2323. Allopurinol Hypersensitivity Syndrome (AHS), A Variant of Drug Reaction With Eosinophilla and Systemic Symptoms (DRESS) Symptoms Cutaneous rash Fever Renal dysfunction Eosinophilia Hepatitis Leukocytosis Death 92% 87% 85% 73% 68% 39% 21% Epidemiology Median dose 300 mg (200-900) Median therapy duration 3 weeks (1-30) Prior renal dysfunction 81% Asymptomatic hyperuricemia 50% Concomitant thiazide diuretic 40% The DRESS syndrome usually commences symptomatically 1 to 8 weeks after exposure to the responsible drug. The symptom complex can be severe. The classic combination is rash, fever, and major internal organ involvement (most commonly hepatitis, but also can include nephritis and pneumonitis). The most common drugs inducing the DRESS syndrome include allopurinol, carbamazepine, phenobarbitol, phenytoin, minocycline, dapsoen, and sulfonamides. Hande KR, et al. Am J Med. 1984;769(1):47-56 Approximate Prevalance of the Human Leukocyte Antigen (HLA) Allele HLA-B*5801 in Various Geographic Regions of the World Unshaded areas represent regions where prevalence of the gene has not been determined. Middleton D, et al. Tissue Antigens. 2003;61(5):403-407 Risk Factors for Allopurinol Hypersensitivity Reaction Risk Factor Recent initiation of allopurinol Renal impairment Diurectic therapy HLA-B*5801 Allopurinol dose (positive association) Allopurinol dose (negative association) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Reference(s) 1,2,3,4 1,2,4,5,6,7 1,2,5,6,7 4,8 1,2,5,9 4,10,11 Hande KR, et al. Am J Med. 1984;76:47-56 Lupton GP, et al. J Am Acad Dermatol. 1979,1:365-374 Singer JZ, et al. Arthritis Rheum. 1986;29:82-87 Hung SI, et al. Proc Nat Acad Sci USA. 2005,102:4134-4139 Arellano F, et al. Ann Pharmacother. 1993;27:337-343 Lang PG Jr, South Med J. 1979,72:1361-1368 Young JL Jr, et al. Arch Intern Med. 1974;134:553-558 Zinch I, et al. Pharmacogenomics. 2011,12:1741-1749 Perez-Ruiz F, et al. J Clin Rheumatol. 2005,11:129-133 Dalbeth N, et al. J Rheumatol. 2006;33:1646-1650 Vazquez-Mellado J, et al. Ann Rheum Dis. 2001;60:981-983 Dalbeth N, et al. Semin Dial. 2007;20:391-395; Zinch I, et al. Pharmacogenomics. 2011,12:1741-1749. Febuxostat and Allopurinol Febuxostat Allopurinol CH3 Structure H3C H O N N NC S CH3 CO2H Tablet Formulation Dosing Range Dosing Frequency 40 mg or 80 mg 40 mg-80 mg Once daily Drug Elimination Primarily hepatic Dose adjustment in None Patients with mild to moderate renal impairment N N HN O 100 mg or 300 mg 100 mg-800 mg Once daily for ≤300 mg Divided doses for >300 mg Primarily renal Yes Febuxostat Overview • Febuxostat is a xanthine oxidase (XO) inhibitor indicated for the chronic management of hyperuricemia in patients with gout • Febuxostat is not recommended for the treatment of asymptomatic hyperuricemia • Febuxostat may reduce elevated sUA in chronic gout patients who: - Have sUA >6 mg/dL on existing therapy - Are still flaring - Have mild to moderate renal impairment* There are insufficient data in severe renal impairment (ClCr <30 mL/min). Caution should be exercised in those patients. Febuxostat is contraindicated in patients being treated with azathioprine or mercaptopurine. Febuxostat Dosing and Administration Usage Dosing • 40 mg is the recommended starting dose Once daily • 80 mg is the recommended if sUA < 6 mg/dL is not achieved after 2 weeks with 40 mg dose Once daily • Upon initiation of treatment, prophylaxis with and NSAID or colchicine may be beneficial Up to 6 months An increase in gout flares is frequently observed during initiation of anti-hyperuricemic agents, including Febuxostat. If a gout flare occurs during treatment, Febuxostat need not be discontinued. Febuxostat Dosing Considerations Special Considerations Dose Adjustment • Febuxostat can be used in patients with mild 0 to moderate renal or hepatic impairment • Febuxostat can be used in patients receiving 0 certain common medications – Warfarin – Hydrochlorothiazide – Colchicine – Naproxen – Indomethacin – Desipramine Febuxostat Efficacy: APEX, Fact, and Confirms Proportion of Patients With sUA <6 mg/dL at Final Visit Patients, % APEX (6 months) 80 70 60 50 40 30 20 10 0 FACT (1 year) 74%* 72%* (n=253/10) Febuxostat Allopurinol 80 mg 300/100 mg 67%*† 45% 39% (n=253) CONFIRMS (6 months) 42% 36% (n=249) (n=242) Febuxostat Allopurinol 80 mg 300 mg *P<0.001 vs allopurinol; †P<0.001 vs Febuxostat 40 mg. (n=757) (n=756) (n=610/145) Febuxostat Febuxostat Allopurinol 300/200 mg 80 mg 40 mg Confirms Efficacy in Patients With Mild to Moderate Renal Impairment Proportion of Patients With Mild to Moderate Renal Impairment with sUA <6 mg/dL at Final Visit Confirms (6 months) 80 Patients, % 70 72%* 60 50 40 50%* 42% 30 20 10 0 *P≤0.05 vs allopurinol. (n=479) (n=503) (n=356/145) Febuxostat 40 mg Febuxostat 80 mg Allopurinol 300/200 mg Renal impairment was defined as baseline estimated ClCr ≥30 mL/min and <90 mL/min. Adverse Reactions Summary: Physician-Reported Placebo Adverse Reactions (≥1% of Patients*) (n=134) Febuxostat Febuxostat 40 mg 80 mg (n=757) (n=1,279) Allopurinol 300/200/100 mg (n=1,277) Liver Function Abnormalities 0.7% 6.6% 4.6% 4.2% Nausea 0.7% 1.1% 1.3% 0.8% 0% 1.1% 0.7% 0.7% 0.7% 0.5% 1.6% 1.6% Arthralgia Rash *At a ≥0.5% greater rate than with placebo. Probenecid • ColBenemid • Can impair renal secretion of : • Inhibits tubular - Sulfinpyrazone, reabsorption of filtered - Sulfonylureas urate in kidney - Indomethacin • Controls hyperuricemia - Penicillin and prevents tophus - Sulfonamides formation • Salicylates interfere with action • Use caution with decreased renal function Drugs Potentially Affected by Probenecid Therapy • Ampicillin • Indomethacin • Penicillin • Heparin • Nafcillin • Dapsone • Cephradine • Methotrexate • Cephaloridin • Rifampicin • Salicylates Uricase Enzymes Uricase Uricase H 2O + O2 H2O2 + CO2 OH N N HO OH N N H OH OH N N N N OH HO N Uric acid N H OH HO N N H Allantoin Uricase (uric acid oxidase) catalyzes the eventual conversion of uric acid to allantoin, a more soluble, readily renally excreted form. Uric Acid Production • About two-thirds of uric acid is generated endogenously by the body, while one-third comes from purines in the diet Xanthine Oxidase Urate Oxidase (Uricase) Xanthine Oxidase Purine Catabolism2-5 Hypoxanthine Xanthine Uric Acid End product for humans, higher primates, reptiles, birds, and some mammals Allantoin End product for the majority of mammals Reduction in Plasma Urate Levels With IV Pegloticase in Phase 3 Trials Mean PUA (All subjects) Mean PUA (mg/dL) (SE) 14 Placebo Pegloticase 8 mg q 2wk 12 10 8 6 4 2 0 0 3 6 9 12 15 18 21 24 27 Week Replicate multicenter, phase 3, double-blind trials assigned to 128 patients with sever, refractory gout to receive either pegloticase 8 mg q2w, or a placebo infusion (n=43). The trials’ primary end point was a sustained plasma urate of <6.0 mg/dL in months 3 & 6. There is evidence for loss of efficacy overall overall over time in the treatment group. Reduction in Plasma Urate Levels With IV Pegloticase in Phase 3 Trials Mean PUA (Responders) Month 3 Placebo Nonresponders Responders Mean PUA (mg/dL) 14 12 Month 6 10 8 6 4 2 0 1 3 5 7 9 11 13 15 17 19 21 23 25 Week Data are for those subjects in the q2w treatment arm of the phase 3 trials separating responders (ie, those who achieved the defined primary end point) vs nonresponders. Nonresponders were generally those subjects who developed high titers of pegloticase antibodies, and these antibodies correlated with infusion reactions. Pegloticase-Associated Infusion Reaction Relationship to Pre-infusion Serum Urate Level IRs per 100 Infusions 6 5 5.5 RCT OLE 4.8 4 3 2 1 0 0.8 0.5 <6 mg/dL >6 mg/dL Data from phase 3 randomized controlled trials (RCT) and open-label extensions (OLE) demonstrate a marked relationship between an increased frequency of infusion reactions (IR) and the loss of effectiveness of pegloticase. For all infusions after the initial pegloticase infusion, the likelihood of IRs depends greatly on whether the preinfusion serum urate determination is above or below 6.0 mg/dL. Most Common Signs and Symptoms of Infusion Reaction to Pegloticase* • • • • • Chest discomfort Flushing Dyspnea Nausea / vomiting Back / flank pain • Erythema • Blood pressure changes • Muscle spasm / stiffness • Hyperhidrosis *Data include all subjects in RCT and OLE in biweekly, monthly, and placebo arms. Strategy for Lowering Uric Acid • Initiate prophylaxis with low dose Colchicine or NSAID 1-2 weeks prior to urate lowering agent • Initiate uricostatic agent at low dose (100 mg/day Allopurinol or 40 mg/day for febuxostat) • Escalate dose every 2-4 weeks while monitoring for toxicity until serum urate is < 6.0 mg/dL • If treat to target goal achieved maintain antiinflammatory prophylaxis for 3-6 months after last gouty flair or longer when tophi persist Strategy for Lowering Uric Acid • Maintain urate-lowering agent indefinitely and check serum urate levels every 6-12 months • If toxicity occurs with one uricostatic drug try the other agent. No evidence of crossreactive toxicity. • If target serum urate not achieved with uricostatic agent, a uricosuric can be added and titrated or Pegloticase can be tried • No adjustment during gout flares Study Showed Maintaining sUA <6 mg/dL Is Associated With Reduced Risk of Future Gout Attacks Incidence of Recurrent Gout Attack, % Incidence of recurrent gout attacks more than 1 year after each patient visit* 80 86% of patients who achieved a serum uric acid level of <6 mg/dL (n=81) had no gout attacks during the observation period 60 40 Observed Logistic regression (n=91) 20 0 10.0 9.5 9.0 8.5 8.0 7.5 7.0 6.5 6.0 5.5 Average Uric Acid Level During Investigation Period (mg/dL) • Based on a retrospective analysis of 267 predominantly male gout patients for up to 3 years (Tokyo, Japan). Baseline mean sUA level >7.45 mg/dL. Urate-lowering therapies prescribed: allopurinol and benzbromarone. Tracking sUA Levels sUA Levels Over Time sUA Level Intercritical Period Gout Attack Intercritical Period • The best time to measure sUA is after a flare has resolved, at least 2 weeks postflare - sUA levels may be artificially low ~50% of the time during a flare • Evaluate sUA in patients on therapy to ensure that the target level is achieved and maintained - ACR guidelines recommend monitoring sUA level every 2-5 weeks during urate-lowering therapy titration, then every 6 months once sUA target has been achieved ACR=American College of Rheumatology. Limitations of Lifestyle Modifications on Reaching Target sUA Levels • sUA levels may be reduced by making lifestyle changes including: - Losing weight Limiting consumption of purine-rich meat and seafood Reducing alcohol intake, particularly beer Limiting high-fructose corn syrup intake Consuming dairy products Medication prescription and monitoring of SUA in patients with a diagnosis of gout n/N % 297/643 46 SUA checked and reached target 126/643 20 SUA checked but did not reach target 127/643 20 No SUA check 390/643 61 Continuous allopurinol prescription n/N % SUA monitoring Colchicine/NSAID prophylaxis Colchicine Colchicine or NSAID ≥14 days before new allopurinol prescription 66/643 10 166/643 26 Before new allopurinol prescription 80/643 12 191/643 30 On or before new allopurinol prescription 169/643 26 311/643 48 ≤14 days after new allopurinol prescription 180/643 28 329/643 51 Singh JA, Jodges JS, Asch SM. Opportunities for improving medication use monitoring in gout. Ann Rheum Di. 2009;68:1265-1270 Take-Away Messages • The goal of therapy in subjects with multiple recurrent gout flares or tophaceous gout is to prevent disease progression by reducing the body urate burden. • Any patient presenting with advanced gout, or simply demonstrating tophi on physical exam, should clearly be started on ULT. • Both allopurinol and febuxostate are effective in gout patients whose hyperuricemia is caused by either uric acid overproduction or underexctetion. • Uricosurics should not be used in overproducers of uric acid or as monotherapy in those with a history of urolithiasis. Take-Away Messages • The uricostatics can be administered as once-daily medications, which increases compliance. Probenecid requires 2 to 3 doses per day for optimal function. • The uricostatic agents function well in the face of renal insufficiency, whereas probenecid requires a GFR of at least 50 mL/min to be most effective. In fact, febuxostat appears to function better than many other options in subjects with mild-tomoderate renal insufficiency. Take-Away Messages • Rash will develop in at least 2% of patients started on allopurinol. Most rashes are benign and dose related, but allopurinol is one of the more common drug causes of severe cutaneous reactions in the form of SJS or TEN and rashes as a component of the DRESS syndrome, which is more commonly known as AHS. • HLA-B*5891, which is by far most common in those of Southeast Asian ancestry, is a marked risk factor for severe allopurinol cutaneous reaction particularly in Koreans with CKD, and those of Han Chinese and Thai descent. Take-Away Messages • In essence, the appropriate strategy for ULT is termed “treat to target,” with the evidence-based target to begin to achieve superior outcomes being achievement of a serum urate of <6.0 mg/dL, at a minimum. This requires checking the serum urate level regularly (eg, every 6 months once the target has been achieved). • In patients with one or more tophi detected on physical exam, the authors prefer a serum urate target of <5.0 mg/dL and in certain patients with advanced disease and multiple tophaceous deposits, lowering the serum urate level to <4.0 mg/day is the preferred strategy by the authors to help optimize therapy outcome. Take-Away Messages • In certain patients with advance disease and multiple tophaceous deposits, lowering the serum urate level to well below 6.0 mg/dL, such as in the range of 4.0 mg/dL, appears to help optimize therapy by accelerating resolution of tophi. • The recombinant PEGylated uricase pegloticase is FDA-approved as in approach for potent and rapid reduction of serum urate and achieves rapid debulking of tophi (within 6 months) in drug responders (which were between 40% to 50% of subjects in phase 3 clinical trials, using 8 mg IV every 14 days). Take-Away Messages • The therapeutic program of urate-lowering measures, once initiated, should keep the serum urate less than 6.0 mg/dL (at a minimum) for the remainder of the patient’s life, even after tophi and gouty arthritis attacks are no longer present. Uric Acid Overproducers and Underexcretors Uric Acid Underexcretion Urate Overproduction (~90%) (~10%) Primary Hyperuricemia • Disordered urate transport • Metabolic disorders Secondary Hyperuricemia • Impaired renal function with urate transport • Excessive purine intake • Drug-induced renal toxicity • Idiopathic • Tumor lysis syndrome Gout Flares Occur When Crystals Trigger an Acute Inflammatory Response Hyperuricemia • Acute flares may be triggered by fluctuations in sUA levels, which can mobilize crystals Supersaturation • Crystals released into the joint space undergo phagocytosis Crystal Formation • Phagocytosis can initiate a proinflammatory response, resulting in gout flares Microcrystal Release Recurrences Inflammatory Cascade Gout Flare