Dr. Jennifer Pearlman
51st Annual Scientific Assembly
Grants/Research Support: none
Speakers Bureau/Honoraria: none
Consulting Fees: none
Other Payments or Relationships with
commercial interests: none
This program has received NO financial
support from any commercial or for-profit
entity
This program has received NO in-kind support
from any commercial or for-profit entity
Potential conflict of interest: none
•
Dr. Jennifer Pearlman has received NO support from related
organizations and/or organizations whose product(s) are being
discussed in this program
No potential sources of bias identified
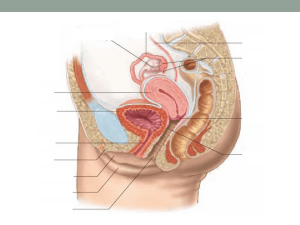
Case Introduction
Defining BioHT
Historical Perspective
Why Consider BioHT
Safety of BioHT
Prescribing BioHT
Conclusion
52 yr, FMP 18m ago
Corporate executive
Wife, mother, daughter, friend
Hot flashes, night sweats
Poor sleep
Irritable, labile mood
Impaired recall and concentration
Loss of libido
Agents of change
Produced by endocrine
cells in specialized organs
Travel via blood/lymph
Exert effect at distant site
Bioidentical HT:
Molecularly equivalent with same lock-and-key fit with in vivo receptor
Source: Natural v. Synthetic
• Form: Pharmaceutical v. Custom Compounded
• Route: Oral v. Transdermal v. Transvaginal
•
Traditional HT:
Synthetic Hormone Analogues or Non-human equivalent
Outcomes: Cognitive, Bone, Heart
Side Effects: Bleeding, Mood, Sleep, Weight
Risks: Cardiovascular, Clotting, Breast Cancer
Nomenclature confusion
Perceived paucity of evidence
No patents No sponsor
No drug reps/patient leaflet
Political/regulator intervention (i.e. estriol)
Perceived as alternative treatment
Stigma “Suzanne Effect”
1930’s -Collip & McKenna (Ayerst) produce
EMMENIM (urine of pregnant Canadian women)
Reformulated w Stallions, Pregnant mares (T2-3),
potency 2-3x human
1942 -FDA approves Premarin (PREgnant MARes
urINe), depsite known risk EC
1966 -Robert Wilson “Feminine Forever” (Ayerst
$50k research grant)
Sodium Estrogen Sulfate
Mg/tablet
Estrone
0.37
Equilin
0.168
17a-Dihydroequilin
0.102
17a-Estradiol
0.027
17b-Dihydroequilin
0.011
l7a-Dihydroequilenin
0.011
17b-Dihydroequilenin
0.021
Equilenin
0.015
l7b-Estradiol
0.005
D8,9-dehydroestrone
0.026
http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm168836.htm
E1 -Estrone:
•
E2 -Estradiol:
•
made in fat cells from Testosterone by aromatase
most active, abundant, ovarian, stimulates
breast/uterus (ER alpha)
E3 -Estriol:
•
weak estrogen, made by placenta/liver, breast
protective, neuroprotective, specific to vaginal mucosa
Widely approved/used 1st line for urogenital
atrophy (outside NA)
•
Ovestin 0.5mg E3 supp, 1mg cream
•
New ultra low dose 50mcg E3 gel
Currently unavailable in NA:
•
Politicized in US (blocked by FDA), no HC approved Rx
Under Investigation:
•
Clinical trial P3 5mg oral dose in MS
Clin Exp Obstet Gynecol. 2011;38(2):143-5. Chuery AC, Speck NM, de Moura KF, Belfort PN, Sakano C,
Ribalta JC.
OBJECTIVE:
This study evaluates the effect of intravaginal estriol on urogenital atrophy, Pap smear parameters,
colposcopy parameters and discomfort during gynecological examination.
METHODS:
31 postmenopausal women who had not used hormone therapy in the previous six months were studied.
All women used intravaginal estriol, 1 mg/day for 21 days. The following variables were analyzed
before and after treatment: complaints of urogenital atrophy; cytological parameters, colposcopic
parameters, and subjective evaluation of discomfort during gynecologic examination.
RESULTS:
All urogenital atrophy complaints improved after treatment. At the first visit, 45.2% of women presented a predominance of
profound cells, 51.6% with predominance of intermediate cells, and 3.2% with predominance of superficial cells. At the
second visit, these rates were 35.5%, 64.5%, and 0%, respectively. Evaluation of the maturation index showed that 83.9%
of women had atrophic Pap smears, and 16.1% showed good estrogenic level before treatment. At the second visit,
atrophic smears occurred in 12.9%, and 87.1% of women exhibited good estrogenic level (chi2 = 20.045; p = 0.000).
Colposcopy showed that 71% of women had atrophic colpitis and/or petequiae before treatment, while atrophy after
therapy was present in only 6.4%. The evaluation of other colposcopic parameters also improved after treatment. Great
discomfort was reported by 19.4% before and by 0% after treatment.
CONCLUSION:
Intravaginal estriol 1 mg/day for a period of 21 days was efficient in improving
urogenital atrophy, Pap smear parameters and colposcopic evaluation in
postmenopausal women.
Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with
urogenital atrophy: a preliminary study.
Biglia N, et al. Gynecol Endocrinol. 2010 Jun;26(6):404-12.
OBJETIVES:
The study aim is to evaluate the efficacy and safety of two low-dose vaginal estrogen treatments (ETs) and of a nonhormonal vaginal moisturizer in postmenopausal breast cancer survivors with urogenital atrophy.
METHODS:
Eighteen patients receiving estriol cream 0.25 mg (n = 10) or estradiol tablets 12.5 microg (n = 8) twice/week for 12
weeks were evaluated and compared with eight patients treated with polycarbophil-based moisturizer 2.5 g
twice/week. Severity of vaginal atrophy was assessed using subjective [Vaginal Symptoms Score (VSS), Profile
of Female Sexual Function (PFSF)] and objective [Vaginal Health Index (VHI), Karyopycnotic Index (KI)]
evaluations, while safety by measuring endometrial thickness and serum sex hormones levels.
RESULTS:
After 4 weeks, VSS and VHI were significantly improved by both vaginal ETs, with further improvement
after 12 weeks. PFSF improved significantly only in estriol group (p = 0.02). Safety measurements did
not significantly change. Vaginal moisturizer improved VSS at week 4 (p = 0.01), but score returned to
pre-treatment values at week 12; no significant modification of VHI, KI, PFSF was recorded.
CONCLUSIONS:
Both low-dose vaginal ET are effective for relieving urogenital atrophy, while nonhormonal moisturizer only provides transient benefit. The increase of serum
estrogens levels during treatment with vaginal estrogen at these dosages is
minimal.
Progesterone
Precursor to all steroid sex hormones
Made by CL at ovulation
Progestational
Anti-proliferative (breast, uterus)
Crosses BBB (GABA agonist)
Bone Stimulating (osteoblast activation)
Progestins (synthetic analogue)
Androgenic (NETA)
Non-androgenic (MPA= Provera)
•
•
Medroxy Progesterone
Acetate (MPA)
RR Breast cancer: MPA 1.48 v. Progesterone 1.0 (no risk)
RR VTE: MPA 1.8 v. Progesterone 0.9 (10% decrease)
French E3n Cohort Study ,Fournier, Br Ca Res. Treat 2008;107:103-111
18
1940’s –Marker Process discovered
1950’s –MPA developed to be more potent, orally
bioavailable than progesterone
1956 -MPA (Provera) patented, approved
•
Approval based on –ve EB data only (3y, n=596)
1980 -Oral MP (Prometrium) approved in France
1990’s -Upjohn provides MPA & MP for PEPI
1994 -NHI funded WHI begins, using CEE+ MPA
1999 –FDA approves Prometrium in US
Progesterone’s improved cardiovascular safety
profile established prior to WHI enrollment
Estrogen “with cyclic MP has the most
favorable effect on HDL-c and no excess risk of
endometrial hyperplasia”
The Writing Group for the PEPI trial. Effects of estrogen or
estrogen/progestin regimens on heart disease risk factors in
postmenopausal women. The Postmenopausal Estrogen/Progestin
Interventions (PEPI) trial. JAMA. 1995; 273(3)1:199-208.
“The contrasting effects of progesterone and MPA
seem clinically important, inasmuch as MPA is a
widely used progestin in the regimen of HRT”.
Otsuki M, et al. Progesterone, but not MPA, inhibits vascular
cell adhesion molecule-1 expression in human vascular endothelial
cells. Arterioscler Thromb Vasc Biol. 2001; 21(2): 243-8.
Santen, RJ. Risk of breast cancer with progestins: critical assessment of
current data. Steroids 2003; 68:953-64.
Stahlberg C. Increased risk of breast cancer following different
regimens of hormone replacement therapy frequently used in
Europe. Int J Cancer 2004; 109: 721-7.
Fournier, A. Unequal risks for breast cancer associated with different
hormone replacement therapies; results from the E3N cohort study.
Breast Cancer Res Treat 2008; 107 (1): 103-11
“the choice of the progestagen in combined HRT is of
importance regarding breast cancer risk; it could be
preferable to use progesterone”
• E3N French epidemiologic cohort study
• Self-administered questionnaires 1990-2002
• 80,377 postmenpoausal women w. up to 12 yrs f/u
Compared with HT Never-Use
Relative Risk (CI)
Estrogen alone
RR 1.29 ( 1.02-1.65)*
Estrogen + progesterone
RR 1.0 (0.83-1.22)
Estrogen + dydrogesterone
RR 1.16 (0.94-1.43)
Estrogen + other progestins
RR 1.69 (1.50-1.91)*
Fournier A et al. Breast Cancer Res Treat 2008: 107:103
Study
Author(s)
Sample (n) Duration
Findings
KEEPS
Multi-centre,
Harman (2005)
720
36m/5yrs
Compared 0.45mg CEE, 50mcg
estradiol in combinations with
cyclic oral MP 200mg 12d
monthly v placebo
PEPI 1995,
2002
Writing Group
875, age 4564yr
3 yrs
Lipoprotein: MP greatest inc
HDL-c (p0.004)
Bleeding: MP fewer days,
amount and episodes of VB
BP: CEE + MP dec in SBP and
DBP v. MPA
Endometrial: MP spares ET and
preserves favorable effects
HERS JAMA
1998
*funded by Wyeth
2,763, avg age
67yr
4.1y; HERS,
6.8y; HERS2
CEE+ MP superior results.
CEE+MPA did not reduce rate
CHD.
E3N Cohort
(2005, 2008)
Fournier, A
80,377, age 4065yr
12yrs
Estrogen only group had 1.32x
inc risk BC (p=0.93); use of
MP+E eliminated risk (p0.001),
v sig inc risk with MPA RR1.48.
E3N-EPIC: Progestins inc risk
BC (RR= 1.4), reduced with MP
(RR=0.9)
ESTHER,
JACC (2007)
Canonico,
M
271, age 4570yr
Oral but not transdermal E inc
VTE risk. Progestins may be
thrombogenic, MP safer.
No first pass effect (limited effect on lipids, clotting factors,
SHBG and free testosterone, thyroid)
Safety: lower risk VTE, CVA, cholecystitis
decrease in risk BC)
Higher bioavailability (nearly 10-fold)
Choice of formulation (patch, gel)
Convenience (2 patch/week)
Consistent blood levels
(no
Current Estrogen
Therapy
VTE Cases (DVT
& PE), (n=259)
Controls (n=603)
Risk VTE
compared to nonusers (OR 95%
CI)
Oral Estrogen
45
39
4.2 (1.5-11.6)
Transdermal
Estrogen
67
180
0.9 (0.4-2.1)
Canonico M, et al. Circulation 2007; 115:840.
Estrogen*
Oral:
•
Estrace 1mg oral tablet
Transdermal/Topical:
Estradot 25-100mcg patch
• Divigel 0.1% ( 0.25-1mg), Estragel 0.75mg/pump (gel)
•
Progesterone
Prometrium 100mg capsule po/pv
•
Micronized progesterone in peanut oil
*Combined EPT: Progestagen required if systemic ET and intact uterus
Vaginal low-dose Estradiol:
*Systemic ET not adequate or indicated for VVA
•
Tablets: Vagifem (10mcg HS PVx2w then 2/w)
•
Ring: Estring (5-10mcg daily over 12w)
Vaginal Progesterone
•
•
Crinone (4% cream, 45mg 2x/wk)
ENDOMETRIN® 100mg vag insert (ART)
Luteal phase defect
Menorragia/AUB
Fertility- luteal support in ART and RPL
Obx- Preterm Labour prevention
Endometrial Hyperplasia
Menopausal EPT
Ann N Y Acad Sci. 1997 Sep 26;828:291-9.
The endometrial effects of vaginal progesterone have been found to be unexpectedly
reliable. This has led us to suspect that a local direct vagina-to-uterus transport or first
uterine pass effect was the basis of the uterine targeting of vaginal progesterone. After
vaginal administration of progesterone, uterine tissue concentration has
been found to exceed by more than 10-fold the levels achieved by
systemic administration, despite plasma levels in the latter case that were more
than seven times higher. Similar differences in systemic-to-uterine tissue level ratios
have been observed between oral and vaginal administration of danazol. Originally
seen as a pharmacological advantage permitting the uterine targeting of vaginally
administered substances, it is possible that the first uterine pass effect plays a
physiological role in the control of uterine contractile activity through the
prostaglandins contained in the semen.
Pharma BioHT
Drug Industry
Manufactured
Health Canada regulated
API* (authorized)
Fixed dose/formulation
Patented
Drug insert, monograph, ads
*API=active pharmaceutical ingredient
Compounded BioHT
Customized Solution
Small batches
Provincially regulated**
API* (authorized)
No drug insert, monograph,
etc.
**Federal Policy; POL-0051 http://www.hcsc.gc.ca/dhp-mps/compli-conform/gmpbpf/docs/pol_0051-eng.php#a51
Biest: E3 estriol: E2 estradiol
•
•
•
•
•
Ratio: 50:50, 80:20
Dose (0.05-1mg)
Volume (0.1cc-0.5cc)
Base (versabase)
Route: transdermal, transvaginal
Progesterone
Dose: 10-200mg
• Schedule: cyclical, sequential
• Route: Vaginal, Oral
• Format: capsule (SR), supp, cream
•
Labs: FSH, estradiol, progesterone, FAI
Screen: MGM, TVUS (if AUB)
Rx: Pharmaceutical BioHT
Estradot 25mcg patch x2/wk
Prometrium 100mg cap HS 5d/wk
Vagifem 10mcg tab, HS PV 2wk then 2x/wk
Bioidentical hormones are chemically and
biologically equivalent to nature’s
Substantial evidence favours their safety
The hormone, the dose and the route matter
Pharmaceutical BioHT can be safely prescribed
Transdermal estrogens may be preferred to
oral
Compounding may be an appropriate option
Nomenclature matters…educate patients!
Questions?