Dr Andy Shepherd
advertisement

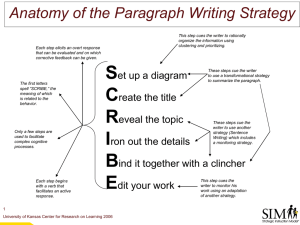
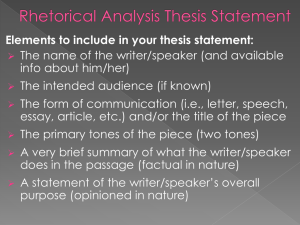
An introduction to Medical Communications Andy Shepherd Senior Medical Writer Who We Are • Global, European, and US full-service medical communications agency • Offices in Oxford, Macclesfield New York, and Sydney New York • ~60 staff of writers, editors, account managers, account directors, graphic designers, conference specialists, and support staff • Established for over 20 years • Enduring client partnerships from early clinical development to lifecycle management including other client partners • Part of the Complete Medical Group Worldwide Network Oxford Sydney Caudex Medical – Part of the Complete Medical Group Worldwide Network Approx 70% of our current business is with Global/HQ clients, 30% with domestic clients Currently: 60 people in North America 290 in Europe 60 in Asia Pac We are in the process of establishing capabilities in France and Latin America 3 Some of our clients… Drug development EMA/FDA review process Phase III Post marketing 1000-5000 patients Phase II 100-300 patients Phase I Preclinical 20-80 healthy volunteers laboratory and animal testing SALES Discovery 0 2 4 6 8 10 12 Patent EMA = European Medicines Agency FDA = (US) Food and Drug Administration 14 16 18 20 years Patent expires What is medical communications? Market and KOL development Creative design Medical writing expertise Multimedia Strategic publication planning International meetings management What is it really? • Getting information about new or existing drugs to the people that use or prescribe them, from the people that make them Pharmaceut ical companies Doctors, nurses, pharmacists, payers, patients What do doctors need to know about the new drug? • • • • • • What is the condition the drug treats? Why should I treat this condition? How does the drug work? Is the drug effective and safe? How is this drug different from the others? Are there any health economic implications? Communicating a major trial Congress abstract, e.g. safety Congress abstract, e.g. efficacy Congress abstract, e.g. sub-analyses Primary publication (efficacy and safety) Congress abstract, e.g. protocol Congress abstract on other analyses Primary publication on other analyses Series of satellite symposia Time Scene-setting reviews Results available Editorial Letters to the Editor Review articles in peer-reviewed journals Highly experienced in publication planning and meetings Projects delivered in 2010 • Communications plans 10 • Publication plans 11 • • • • • • • Papers Abstracts Posters/presentations 88 170 167 Satellite symposia Advisory boards Stand-alone meetings Steering committees 7 5 6 13 Acceptance rates: Abstracts 92% Manuscripts 98% Reviews 100% Ghost-writing: Caudex position statement Caudex is committed to working in accordance with the principles of Good Publication Practice and does not engage in ghost-writing What are we looking for? • • Education • Minimum first degree in medicine, pharmacy or life sciences • A higher degree and/or research experience is advantageous Writing skills • Demonstrated ability to produce high quality, scientifically accurate, copy • • • • A positive, can-do attitude Ability to work independently and as part of a team Well organised and able to plan work effectively Proficient in standard software packages (Word, Excel, PowerPoint, Outlook) Caudex trainee programme • • Trainees are usually recruited in pairs Training syllabus, delivered through: • Formal training sessions (internal/external) • One to one mentoring and feedback • On site experience • Clear objectives for promotion Ongoing training • • Ongoing company and department training for all staff Recent examples: • Medical writing skills • CMPP programme • Values workshops • Drug development process • Copyright law • Software training • Social media Compared to working in a lab Pros... Cons... Medical Writer career structure Medical Director Scientific Director 4 years Scientific Advisor Principal Medical Writer Senior Medical Writer 2 years 2 years 2 years Medical Writer Trainee Medical Writer 1 year • For more detailed information about starting salary and salary scale, please speak to me afterwards • Any further questions about Caudex or MedComms in general, please feel free to contact me: andy.shepherd@caudex.com