Discount Eligible Beneficiaries in Enhanced Alternative Plans
advertisement

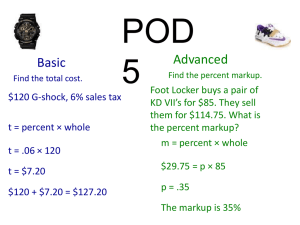
May 2014 Manufacturer Webinar Angela Stanley, CMS, Division of Payment Reconciliation Agenda • CGDP Data Overview • CGDP Appeals • Website and Portal Improvements • An EFT Reminder • Live Q&A 2 CGDP Data Overview Amanda Johnson, Director, Division of Payment Reconciliation Discount Eligible Beneficiary Distribution LICS vs Non LICS Benes Across Benefit Years 40,000,000 35,000,000 30,000,000 25,000,000 Non-LICS Bene Count 20,000,000 LICS Bene Count 15,000,000 10,000,000 5,000,000 0 2011 2012 2013 2014 4 Discount Eligible Beneficiaries in Defined Standard Plans Defined Standard Benefit Plan - LICS vs Non LICS Beneficiary Trend by Benefit Year 3,000,000 2,500,000 2,000,000 Non-LICS Bene Count 1,500,000 LICS Bene Count 1,000,000 500,000 2011 2012 2013 2014 5 Discount Eligible Beneficiaries in Enhanced Alternative Plans Enhanced Alternative Plan -LICS vs Non LICS Beneficiary Trend by Benefit Year 18,000,000 16,000,000 14,000,000 12,000,000 10,000,000 Non-LICS Bene Count 8,000,000 LICS Bene Count 6,000,000 4,000,000 2,000,000 2011 2012 2013 2014 6 Discount Eligible Beneficiaries in Employer Group Waiver Plans EGWP -LICS vs Non LICS Beneficiary Trend by Benefit Year 7,000,000 6,000,000 5,000,000 4,000,000 Non-LICS Bene Count LICS Bene Count 3,000,000 2,000,000 1,000,000 2011 2012 2013 2014 7 Quarter Beneficiaries Enter the Gap 100% 90% 32.1% 29.6% 29.2% 35.0% 35.6% 80% 70% 60% Q4 50% 36.7% Q3 Q2 40% Q1 30% 20% 26.8% 26.2% 6.6% 8.6% 9.1% 2011 2012 2013 24.6% 10% 0% 8 Discount Eligible Beneficiaries by Benefit Phase, Program-wide 25,000,000 661,850 494,864 20,000,000 468,839 3,945,108 3,267,691 3,251,770 15,000,000 Catastrophic Coverage Coverage Gap Initial Coverage Period 10,000,000 Deductible Coverage 5,000,000 0 2011 2012 2013 9 2013 Ending Benefit Phase for EGWP Discount Eligible Beneficiaries 2013 EGWP Deductible ICP Gap Catastrophic 3% 3% 25% 69% 10 2013 Ending Benefit Phase for EA Discount Eligible Beneficiaries 2013 Enhanced Alternative Plan Deductible ICP Gap Catastrophic 0% 3% 14% 83% 11 2013 Ending Benefit Phase for All Other Plan Types Discount Eligible Beneficiaries 2013 All Other Plan Types Deductible ICP Gap Catastrophic 3% 13% 23% 61% 12 Average Gap Discount Amount per Beneficiary Average Gap Discount Amount 2011 $ 603.75 2012 $ 705.56 2013 $ 910.69 13 Coverage Gap Discount Program (CGDP) Appeals Independent Review Entity (IRE) for the Centers for Medicare and Medicaid Services (CMS) 14 Independent Review Entity for CGDP Review appeals of denied disputes 15 CGDP Appeals • Affirm the appeal – (disagree with denial) • Deny the appeal – (agree with denial) 16 File TPA Dispute Correctly Attachment Summary of Dispute Submission Guidance by Reason Code DISPUTE REASON CODE DISPUTE REASON DESCRIPTION D01 Duplicate Invoice Item D02 Closed Pharmacy D03 Not PART D Covered Drug DOCUMENTATION COMMENTS REQUIRED: The SUPPORTING DETAIL REFERENCE NUMBER field must contain the Detail Reference Number (DRN) of the potential duplicate. OPTIONAL: Supporting evidence in the ADDITIONAL INFORMATION field. REQUIRED: The SUPPORTING DATE 1 field should contain the NCPDP closed date. OPTIONAL: Supporting evidence in the ADDITIONAL INFORMATION field. REQUIRED: The ADDITIONAL INFORMATION field should contain supporting evidence explaining the statutory exclusion that applies to the drug. Most PDEs disputed for this reason represent adjustments to previously invoiced items. Manufacturers should confirm that this is not the case by ensuring that the DRN from the disputed PDE and the DRN entered in the Supporting DRN field are different. The date of service on the dispensing event should post-date the date entered as the NCPDP closed date. Manufacturers should confirm that the Service Provider ID in question does not represent a pharmacy for disputes on the basis that the drug is covered under Part B. 17 Know the Guidance DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 DEPARTMENT OF HEALTH & HUMAN SERVICES Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, Maryland 21244-1850 CENTER FOR MEDICARE CENTER FOR MEDICARE DATE: March 5, 2012 TO: Pharmaceutical Manufacturers FROM: Cheri Rice /s/ Director, Medicare Plan Payment Group Medicare Coverage Gap Discount Program—Dispute Resolution SUBJECT: Background As part of the Medicare Coverage Gap Discount Program (CGDP), manufacturers are required to provide applicable beneficiaries with applicable discounts on the negotiated price of applicable drugs. Applicable beneficiaries are Part D enrollees that do not receive income related subsidies under section 1860D-14(a) of the Social Security Act (the Act), have reached or exceeded the initial coverage limit (ICL) under section 1860D-2(b)(3) of the Act, and have not incurred costs for covered Part D drugs in the year equal to the annual out-of-pocket threshold specified in 1860D-2(b)(4)(B) of the Act. TO: Pharmaceutical Manufacturers FROM: Cynthia G. Tudor, Ph.D., Director, Medicare Drug Benefit and C & D Data Group SUBJECT: Medicare Coverage Gap Discount Program Appeals Guidance DATE: May 31, 2011 This memorandum provides manufacturers with the revised guidance for appealing invoiced discount payments under the Medicare Coverage Gap Discount Program (Discount Program). CMS issued the draft guidance on April 7, 2011 for public comment. We reviewed all of the timely comments received and are making a number of changes and clarifications in this guidance. However, not all of the comments are addressed in this guidance because they were beyond its scope. We will consider addressing other comments, such as those pertaining to our low-volume cell policy, in future guidance or rulemaking. • Not calculated correctly • Not calculated with accurate data • Explain why determination is incorrect The Centers for Medicare & Medicaid Services (CMS) generates invoices to manufacturers for applicable discounts based on prescription drug event (PDE) data submitted by the Part D sponsors. Manufacturers have the right to dispute invoiced discount payments based on the “Medicare Part D Discount Information on the Manufacturer Data Report.” Within 60 days of receipt of the information, manufacturers must electronically submit all disputes using the “Manufacturer Dispute Submission File” format provided by the Third Party Administrator (TPA). The PDEs accepted from Part D plan sponsors are saved after a rigorous set of data edits are applied. These include 15 different edits focused exclusively on the CGDP. For example, Edit 867 will reject a PDE submission if the Food and Drug Administration (FDA) data available at the time does not designate the drug as having a New Drug Application (NDA) or a Biologic License Application (BLA). Without these designations the drug is ineligible for the coverage gap discount. Detailed information on PDE submissions, including the editing process, can be obtained on the TPA's website. We specifically recommend reviewing the "2011 PDE Participant Guide.” 1 In addition to the substantive editing process, PDEs with coverage gap amounts are subject to further data analysis by CMS before they are used to generate invoices to the manufacturers for the CGDP. Accordingly, we expect relatively few PDE errors that would require a dispute to correct and will generally deny these types of disputes unless the evidence presented demonstrates a failure in the editing and review process. Moreover, CMS will deny disputes if the discount payment is accurately calculated, even if the dispensing event may not have been clinically appropriate. In other words, the dispute process CMS is implementing an appeals process in accordance with section 1860D-14A(c)(1)(A)(vii) of the Affordable Care Act of 2010 and section V of the Medicare Coverage Gap Discount Program Agreement (the Agreement). Section 1860D-14A(c)(1)(A)(vii) of the Act requires us to provide a reasonable mechanism to resolve manufacturer disputes involving the discounts provided under the Discount Program and section V of the Agreement specifies the rights and obligations of both CMS and manufacturers for resolving such disputes. A copy of the Agreement can be found on the CMS website at: http://www.cms.gov/PrescriptionDrugCovGenIn/05_Pharma.asp#TopOfPage. The guidance specifies the standard that manufacturer appeals must satisfy in order for the independent review entity (IRE) to further review and validate a disputed discount payment. This guidance also clarifies what facts manufacturers must demonstrate, at a minimum, to satisfy this standard. The standard is necessary to help ensure that the appeals process efficiently and effectively addresses potential gap discount payment errors in accordance with statutory and contractual requirements while discouraging the submission of appeals based primarily upon unfounded bases. We will evaluate the implementation of the appeals process, and revise this guidance in the future, if necessary, to address issues that might emerge. 1 Available online at http://www.mcoservice.com/internet/Cssc.nsf/files/PDEParticipantGuide%20cameraready%20081811.pdf/$FIle/PD EParticipantGuide%20cameraready%20081811.pdf -1- 18 Reason Codes • Duplicate Invoice Item • Closed Pharmacy • Not a Covered Part D Drug 19 Reason Codes • Aberrant Quantity • High Price of the Drug • Last Lot Expiration 20 Reason Codes • Marketing Category – Not NDA – Not BLA • Excessive Gap Discount – Single – Cumulative 21 Excessive Gap Discount Single Claim 2013 – TrOOP is $4,750 and the plan covers 2.5% of negotiated drug costs [($4,750÷0.975) X 0.50] 2014 – TrOOP is $4,550 2013 – Maximum discount $2,435.90 2014 – Maximum discount $2,307 22 Excessive Gap Discount Cumulative Claims Depends on secondary payers and their TrOOP status 2013 - maximum discount for multiple claims is $4,871 2014 - decreases to $4,667 23 Appeals Received 24 Appeals by Reason Codes 25 Appeal Outcomes 26 DRN Outcomes 27 Affirmed Examples • Part D Sponsor stated the PDEs would be adjusted – Incorrect calculation – Incorrect NDC – Incorrect quantity – Incorrect data submitted • Exceeded maximum discount • Not Part D Covered Drug 28 Denied Examples • Supporting evidence absent – Non covered drugs – Excessive gap discount – Inappropriate doses – Excessive cost • Prior Authorization present 29 Conclusion • File your dispute correctly • File your appeal timely • Respond to communications received from the IRE CGDPAppeals@provider-resources.com 30 CGDPAppeals@ provider-resources.com 31 Website and Portal Improvements Art Spaziano, Manager Palmetto GBA Medicare Part D, CGDP TPA Website and Portal Improvements • TPAdministrator.com – FAQ – ListServ Announcement History • Manufacturer’s Portal – Payment Confirmation • Support for negative amounts – Detail reports – Confirmation responses • Sponsor Mailboxes – Web based payment confirmation 33 TPAdministrator.com FAQ 34 TPAdministrator.com FAQ 35 TPAdministrator.com ListServ Announcement History 36 Manufacturer’s Portal Bi-directional Pymt Confirmation 37 Manufacturer’s Portal Detail Reports 38 Manufacturer’s Portal Confirmation Responses 39 EFT Reminder Art Spaziano, Manager Palmetto GBA Medicare Part D, CGDP TPA EFT Information for Manufacturers and Sponsors • It’s the only way you can receive what you’re owed • No Dependency on APPS system • Opportunity to update each quarter • No update needed if there are no changes • Reminders will be sent prior to each update deadline • Full set of EFT records redistributed each quarter • Important for Manufacturers & Sponsors to download their respective files each quarter • Granular description of changes issued with each update • EFT info for some DMs still missing! 41 Contact Information Art Spaziano, Manager Palmetto GBA Medicare Part D, CGDP TPA Contacting the TPA • TPA website - http://tpadministrator.com • Phone – Help Line: 1-877-534-2772 – Option 1 – Hours: Monday thru Friday 8am to 7pm ET • General email inquiries regarding the invoicing and payment process should be sent to tpaoperations@tpadministrator.com • Webinar slides will be posted to the TPA website • Suggestions for future webinar topics should be sent to webinar@tpadministrator.com • Questions related to dispute files, EFT information, invoice corrections http://tpadministrator.com – Website • disputes@tpadministrator.com - Dispute support documentation 43 Resources • Medicare Drug Benefit Group • Questions related to the Manufacturers Agreement, changes of ownership, terminations, compliance / administrative-related issues, policy • Email address is CGDPandManufacturers@cms.hhs.gov • Discount Program Manufacturer’s Page • http://www.cms.gov/Medicare/Prescription-DrugCoverage/PrescriptionDrugCovGenIn/index.html?redirect=/PrescriptionDrugCov GenIn/ • HPMS Website Updating CMS contact and labeler code changes • https://gateway.cms.gov - Website • CMS_IT_service_desk@cms.hhs.gov - Password Resets • CMSHPMS_access@cms.hhs.gov - Non-password access assistance • Independent Review Entity (IRE) for Discount Program appeals. • https://cgdpappeals.provider-resources.com/Default.aspx - Website