Insulin, Glucagon, and Diabetes Mellitus

Insulin, Glucagon, and Diabetes Mellitus
Prof. dr. Zoran Valić
Department of Physiology
University of Split School of Medicine
digestive functions two important hormones: insulin and glucagon (crucial for normal regulation of glucose, lipid, and protein metabolism) other hormones: amylin, somatostatin, and pancreatic polypeptide
1)
2)
acini (secrete digestive juices) islets of Langerhans (secrete insulin and glucagon directly into the blood)
1-2 million islets of Langerhans (0.3 mm in diameter) three major types of cells: alpha, beta, and delta cells
beta cells – 60%, lie mainly in the middle of each islet, secrete insulin and amylin alpha cells – 25%, secrete glucagon delta cells – 10%, secrete somatostatin
PP cells – present in small numbers, secrete pancreatic polypeptide?
insulin inhibits glucagon secretion, amylin inhibits insulin secretion, and somatostatin inhibits secretion of insulin and glucagon
Insulin and Its Metabolic Effects
Banting and Best (1922) – isolated inzulin insulin has always been associated with
"blood sugar“, but it has profound effect on metabolism of fat (acidosis, arteriosclerosis) and proteins insulin secretion is associated with energy abundance
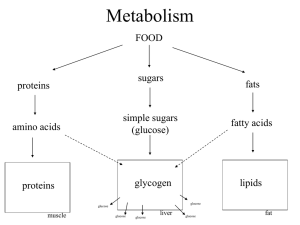
excess amounts of carbohydrates cause increase in insulin secretion insulin stores the excess carbohydrates as glycogen (liver and muscles) inzulin stores adipose tissue inzulin promotes amino acid uptake by cells and conversion of amino acids into protein inzulin inhibits the breakdown of proteins
Insulin Chemistry and Synthesis
small protein (5808) it is composed of two amino acid chains connected by disulfide linkages spliting of the chains – loss of activity translation of the insulin RNA by ribosomes
preproinsulin (11500)
in the ER proinsulin (9000)
in Golgi apparatus insulin and the C chain peptide
circulates almost entirely in an unbound form plasma half-life averages only about 6 min cleared from circulation within 10 to 15 min degraded by the enzyme insulinase mainly in the liver, to a lesser extent in the kidneys and muscles
Activation of Target Cell
Receptors and Resulting Effects
activation of membrane receptor protein autophosphorylation of beta subunits
activation of tyrosine kinase
phosphorylation of multiple other intracellular enzymes including a group called insulin-receptor substrates (IRS)
after few seconds 80% of cells increase their uptake of glucose (muscle and adipose cells) cells become more permeable for many amino acids, potassium and phosphate ions within 10-15 min change in activity of many intracellular metabolic enzymes within few hours or days – rates of translation and transcription
Inzulin
Muscle Glucose
Uptake and Metabolism
1)
2) muscle tissue depends on fatty acids, resting muscle membrane is only slightly permeable to glucose moderate or heavy exercise – fibers more permeable in absence of insulin few hours after a meal (BGC is high and pancreas is secreting large quantities of insulin)
if the muscles are not exercising after a meal, glucose is stored in the form of muscle glycogen (2-3%) spurts of anaerobic energy insulin facilitates glucose transport through the muscle cell membrane
Insulin
Liver Uptake, Storage, and Use of Glucose
1)
2)
3)
storing most of glucose in the form of glycogen after a meal and its release between two meals inactivates liver phosphorylase (glikogen) increasing the activity of glucokinase
(initial phosphorylation of glucose) increase activity of glycogen synthase about 5-6% of the liver mass (100g)
Glucose Release Between Meals
1)
2)
3)
4) pancreas decrease its insulin secretion stopping synthesis of glycogen and preventing uptake of glucose in the liver lack of insulin & increase of glucagon activates phosphorylase (splitting of glycogen into glucose phosphate) activation of glucose phosphatase (spliting of phosphate radical, diffusion of glucose)
insulin promotes conversion of excess glucose into fatty acids (lipoproteins – fat) inzulin inhibits gluconeogenesis (decreasing the quantities and activities of the liver enzymes and availability of precursors required for gluconeogenesis)
Lack of effect of insulin on glucose uptake and usage by brain
most of the brain cells are permeable to glucose and can use glucose without the intermediation of insulin only glucose for energy when the BGC falls too low (1-3 mmol/L) – hypoglycemic shock (nervous irritability, fainting, seizures, and even coma)
Effect of Insulin on Fat
Metabolism
not quite as visible, but equally important long-term effect of insulin lack – extreme atherosclerosis (MI, cerebral strokes) insulin promotes fat synthesis and storage increases the utilization of glucose (fat sparer) promotes fatty acid synthesis (in liver):
1)
2)
3) insulin increases transport of glucose into liver cells (glucose
pyruvate
acetyl-
CoA
fatty acids) excess of citrate and isocitrate ions formed by citric acid cycle when excess amounts of glucose are being used for energy
activation of acetyl-CoA carboxylase formation of triglycerides and release from liver cells in form of lipoproteins; insulin activates lipoprotein lipase in adipose tissue
Storage of Fat in Adipose Cells
1)
2) insulin inhibits the action of hormonesensitive lipase (hydrolysis of the triglycerides in fat cells) insulin promotes glucose transport through the cell membrane into the fat cells; forms large quantities of α-glycerol phosphate
(base for glycerol)
Insulin Deficiency Increases Use of Fat for Energy
hormone-sensitive lipase in the fat cells becomes strongly activated (release of fatty acids and glycerol into circulating blood) increases plasma cholesterol and phospholipid concentrations (3x increase in plasma lipoproteins) ketosis and acidosis (acetoacetic acid – acidosis, β-hydroxybutyric acid & acetone)
Effect of Insulin on Protein
Metabolism and on Growth
3)
4)
5)
1)
2) insulin stimulates transport of many amino acids into the cells (valine, leucine, isoleucine, tyrosine, and phenylalanine) increases translation of mRNA ("turns on" the ribosomal machinery) increases transcription of DNA (enzymes) inhibits catabolism of proteins (lysosomes) depresses gluconeogenesis (enzymes)
insulin and growth hormone interact synergistically to promote growth insulin is essential for growth two hormones function synergistically to promote growth each promotes cellular uptake of a different selection of amino acids, all of which are required for growth
Mechanisms of Insulin Secretion
response to increased BGC glucose transporters (GLUT 2) in beta cells glucose is phosphorylated to glucose-6phosphate by glucokinase
“rate limiting step”
ATP is formed – inhibits K
ATP channels
(sulfonylurea) – opening Ca channels – exocytosis of inzulina
Control of Insulin Secretion
formerly, it was believed that insulin secretion was controlled almost entirely by
BCG
increased BGC stimulates insulin secretion at BGC 4,5-5,0 mmol/L (80-90 mg/100 ml)
– lučenje 25 ng/min/kg increase in BGC insulin secretion increases markedly in two stages
feedback relation between BGC and insulin secretion rate
BGC of 20-30 mmol/L (400-600 mg/100 ml) insulin secretion is reaching a peak (10-
25x basal level)
Other Factors That Stimulate
Insulin Secretion
1)
2)
3) amino acids (arginine & lysine; little alone, but combined with BGC x2; important) gastrointestinal hormones (gastrin, secretin, cholecystokinin, GIP; amplify the action of glucose; anticipatory effect) other hormones and autonomic nervous system (glucagon, hGH, cortisol, progesterone and estrogen; prolonged secretion – exhaustion of beta cells)
Switching Between Carbohydrate and Lipid Metabolism
insulin promotes use of carbohydrates for energy, depresses the utilization of fats control – BGC hGH, cortisol – are secreted in response to hypoglycemia, inhibit cellular utilization of glucose while promoting fat utilization epinephrine – increasing both (BGC and fatty acids)
Glucagon and Its Functions
alpha cells of the islets of Langerhans increase BGC large polypeptide (3485); composed of a chain of 29 amino acids hyperglycemic effect/hyperglycemic hormone
Effects on Glucose Metabolism
breakdown of liver glycogen
(glycogenolysis; adenylyl cyclase
cAMP
protein kinase
phosphorylase b
phosphorylase a
degradation of glycogen into glucose-1-phosphate
dephosphorylation
BGC; amplifying mechanism)
Effects on Glucose Metabolism
increased gluconeogenesis in the liver
(increase in the rate of amino acid uptake by the liver cells and then the conversion of many of the amino acids to glucose by gluconeogenesis (pyruvate
phosphoenolpyruvate))
Other Effects of Glucagon
activation of adipose cell lipase inhibition of storage of triglycerides in liver
in high concentrations : enhances the strength of the heart, increases blood flow in some tissues, especially the kidneys, enhances bile secretion, inhibits gastric acid secretion
Regulation of Glucagon Secretion
increased BGC inhibits glucagon secretion
(exactly opposite from effect of insulin) increased blood amino acids stimulate glucagon secretion (same as insulin; alanine and arginine) exercise stimulates glucagon secretion (4-
5x, prevents a decrease in BGC)
Somatostatin Inhibits Glucagon and Insulin Secretion
1)
2)
3) delta cells, 14 amino acid polypeptide, extremely short half-life, extend the period of absorption, GHIH in hypothalamus locally, depress secretion of both insulin and glucagon decreases the motility of the stomach, duodenum, and gallbladder decreases both secretion and absorption in the gastrointestinal tract
Importance of BGC Regulation
glucose is the only nutrient used by brain, retina, and germinal epithelium of gonads most of the glucose between meals is used in the brain glucose can exert high osmotic pressure
(cellular dehydration), loss of glucose in urine, osmotic diuresis by the kidneys (loss of fluids and electrolytes), damage to many tissues, especially to blood vessels
Diabetes Mellitus
syndrome of impaired carbohydrate, fat, and protein metabolism, caused by either lack of insulin secretion or decreased sensitivity of the tissues to insulin
two general types : type I – insulin-dependent diabetes mellitus
(IDDM) type II – non-insulin-dependent diabetes mellitus (NIDDM, insulin resistance)
metabolism of all main foodstuffs is altered prevent the efficient uptake and utilization of glucose by most cells of the body increase in BGC cell utilization of glucose falls increasingly lower, and utilization of fats and proteins increases
Type I Diabetes
injury to beta cells or diseases that impair insulin production (viral infections or autoimmune disorders, heredity) usual onset of type I diabetes occurs at about 14 years of age – juvenile diabetes mellitus may develop abruptly (few days or weeks)
increase in BGC to 17-66 mmol/L (300-
1200 mg/100 ml : loss of glucose in urine (BCG > 10 mmol/L) cell dehydration (osmotic transfer of water out of the cells; osmotic diuresis
extracellular dehydration) chronic increase – tissue injury(MI, stroke, kidney failure, retinopathy ,blindness, and ischemia and gangrene of the limbs, peripheral neuropathy, autonomic nervous system dysfunction)
severe metabolic acidosis (excess keto acids) + dehydration = severe acidosis
diabetic coma
death physiological compensations: rapid and deep breathing, decrease in bicarbonate depletion of body's proteins (asthenia (lack of energy) despite eating large amounts of food (polyphagia))
Type II Diabetes
diminished sensitivity of target tissues to the metabolic effects of insulin – insulin resistance far more common (90-95%) occurs after age of 30 – adult-onset diabetes in recent years – younger individuals
(increasing prevalence of obesity)
increased plasma insulin concentration
(hyperinsulinemia) compensatory response by the pancreatic beta cells in the early stages of the disease – moderate hyperglycemia; in the later stages – beta cells become "exhausted" or damaged – more severe hyperglycemia
gradual process, beginning with excess weight gain and obesity (fewer insulin receptors or abnormalities of the signaling pathways) in some individuals pancreas is “used up” in the beginning – limitation of food intake, decrease in body weight
1)
2)
3)
4)
5)
“metabolic syndrome” obesity, especially accumulation of abdominal fat insulin resistance fasting hyperglycemia increased blood triglycerides and decreased blood highdensity lipoprotein-cholesterol hypertension
drugs that increase insulin sensitivity – thiazolidinediones drugs that suppress liver glucose production
– metformin drugs that cause additional release of insulin by the pancreas – sulfonylureas in the later stages of type II diabetes, insulin administration is usually required
Physiology of Diagnosis of DM
urinary glucose (qualitative & quantitative tests)
BGC (4,5-5,0 mmol/L – normala, 6,0 mmol/L gornja granica) & insulin levels oral glucose tolerance test (OGTT) – 1 g glucose / kg body mass acetone breath
Treatment of Diabetes
insulin is available in several forms
(regular, longer-acting insulins) individualized pattern of treatment dieting and exercise animal / recombinant DNA lipid-lowering drugs to control high level of blood cholesterol
Insulinoma – Hyperinsulinism
excessive insulin production from an adenoma of an islet of Langerhans much rarer than diabetes
10-15% of these adenomas are malignant – tremendous production of insulin more than 1 kg of glucose have had to be administered every 24 hours
Insulin Shock and Hypoglycemia
insulin-secreting tumors or administration of too much insulin
BGC: 3-4 mmol/L – CNS becomes excitable, hallucinations, extreme nervousness, trembling, sweating
BGC: 1-3 mmol/L – clonic seizures and loss of consciousness state of coma, permanent damage