Public Reporting of Cardiovascular Data
advertisement

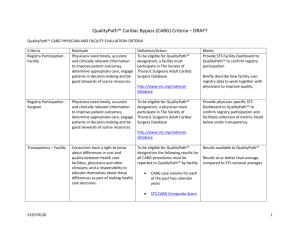
Overview Public Reporting Cardiovascular Data Recommendations Healthcare providers need reliable quality and comparative performance information to advance their quality improvement efforts. Consumers need reliable information to make informed decisions about their care. Comparative Effectiveness What is being compared What are the metrics of comparison Who is performing the comparison National Cardiovascular Data Registry 1998 Cath PCI Registry 1380 sites 11 million patients 2005 ICD Registry 2008 1590 sites Action Registry >600K patients 656 sites 225K patients Pinnacle 800 sites/ 1.9 million patients 2012-future Structural Heart “TAVR” Atrial Fibrillation STS National Database 1990 Adult Cardiac Surgery Database 1994 Adult Aortic and Mitral Valves Adult Thoracic 2002 Adult Congenital Heart Surgery > 90 % of Cardiothoracic Surgery Practices participate in database Important Feedback for Program Results NCDR Executive summary of quarterly institutional outcomes reports and on the dashboard, metrics and measures provides information about quality of care. These metrics and measures represent the most important processes and outcomes of care with a strong link to evidence and clinical guidelines STS The star rating calculation begins by assuming all providers are average and then determines statistically if there is at least a 99 percent probability that the performance of any specific provider is lower than average (one star) or higher than average (three star) Both registries are risk adjusted data ACC-NCDR Blank Data Collection Form Sample Report Complexity of Public Reporting Healthcare providers Consumers Have a track record as effective champions for performance excellence and support public reporting in principle. May be misled by findings of a report or by media interpretation (e.g. Healthgrades where only Medicare billing data is compiled) Invest significantly in the data abstraction necessary for report card formulation. However, much variability in coding of complications (not concurrent). Significant concerns exist over how inter-rater reliability will be achieved. Could have difficulty understanding clinical jargon and graphical interpretation of data. (oversimplification can be misleading as well) Are concerned with impact public reporting will have on public health (as physicians will become risk averse) Can be confused by lack of report card standardization and receive conflicting information Understand that report cards are a snapshot of care and that there is no “perfect” report card May find websites difficult to navigate and have difficulty in accessing information about methodology and limitations of a report card ACC-AHA,AHRQ Public Reporting Consensus Statement Risk adjusted Timely Sufficient in sample size Increase value to consumers and providers Include a relevant time period Easy to use Provide explanations and methodology Above all information must be valid and reliable 1. 2. Krumholz et al, Standards for Statistical Models Used for Public Reporting of Health Outcomes. An American Heart Association Scientific Statement From the Quality of Care and Outcomes Research Interdisciplinary Writing Group. Circulation 2005 doi:10.1161/CIRCULATIONAHA.105.170769 Hibbard J, Sofaer S. Best Practices in Public Reporting No. 2: Maximizing Consumer Understanding of Public Comparative Quality Reports: Effective Use of Explanatory Information. AHRQ Publication No. 10-0082-EF, May 2010, Public Reporting Recommendations Supportive of transparency and public reporting Participate in a federally accredited PSO Only requested risk adjusted outcomes data provided Require a valid inter-rater reliability process Mandatory vs. voluntary reporting STS presently physician owned data and would require further processing Public Reporting Principles Risk Adjusted data only Focus on vital few vs useful many No more than 3-5 measures Clear and Concise Establish a valid Inter-rater reliability process Timely (concurrent abstracting or within 6 months) What ACC measures are recommended? Door to Balloon times PCI inhospital risk adjusted mortality (all patients) Volumes (significant sample size) Stemi (100) Elective Angioplasties (300) Annual volume numbers only If <100 Stemi– report “inadequate sample size to be reported” If <300 Elective PCI – report “inadequate sample size to be reported” What STS measures are recommended? CABG only (no redo’s) Mortality (as observed/expected) CABG Volumes (significant sample size) Annual volume numbers only If <100 CABG– report “inadequate sample size to be reported” *Indicate programs that provide Heart Transplant services* Questions?