ELECTRONS IN ATOMS
advertisement
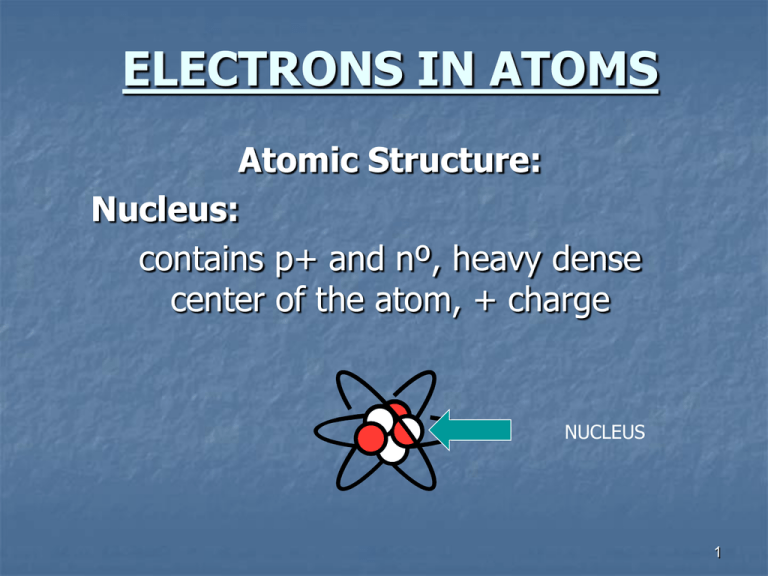
ELECTRONS IN ATOMS Atomic Structure: Nucleus: contains p+ and nº, heavy dense center of the atom, + charge NUCLEUS 1 ELECTRONS Orbit around the nucleus Negatively charge Almost no mass Energy levels (“shells”): are numbered from the nucleus out (inside out). Each consecutive energy level has a larger circumference so it can fit more electrons. (like a stadium) another name for an energy level is principle quantum number n = principle quantum number or energy level # of electrons in the energy level = 2(n2) energy level: # of electrons: 1 )2 2 3 4 )8 )18 )32 *outermost energy level can hold a max of 8eStable Octet – 8 electrons in outermost energy level -most stable electron configuration because it’s the state of least energy. 2 Metals: lose e-, have 1,2 or 3e- in the outer shell Lose 1,2 or 3e- and then the shell underneath has 8e- (stable octet) *exceptions: (Li, Be and B) – Non-metals: lose 1,2 or 3 e- but their shell underneath is the first energy level, which can hold a max of 2e-, and is therefore stable. gain e-, have 5,6 or 7 e- in their outer shell gain 3,2 or 1 e- to achieve a stable octet, (8e-) *exception: hydrogen – is a gas (not a metal), usually loses 1e- to form H+ ion. 3 Subdivisions of Energy Levels in Atoms: (telephone # with extension) - (609) 268-4600 ext. 2667 Principle quantum # = Energy Levels (1,2,3,4) (period # on table = # of energy levels) Sublevels (s,p,d,f) ↓ Orbitals ↓ 2 electrons each max ↓ Opposed Axial Spins Pauli Exclusion Principle: no more than 2e- can occupy an orbital These electrons must have opposite spins 4 Energy levels are subdivided into sublevels Energy Level # = # of sublevels Energy level Sublevels 1 s 2 s,p 1 3 s,p,d s) 4 s,p,d,f Sublevels are divided into 2 sp) 3 sp)d 4 sp)df orbitals___. Each sublevel has a different # of orbitals. s has 1; p has 3; d has 5; and f has 7 Orbital- 3 dimensional region of space with a high probability of finding an electron. (NOT AN ORBIT) Each orbital can hold 2e-max. Think of a fly trapped in a jar! 5 Sublevel s *p d f Orbitals 1 Max. # of electrons 2 6 10 14 3 5 7 *p orbitals are designated as px,py,pz Hund’s rule: When electrons occupy orbitals of equal energy (entering orbitals of the same sublevel) they enter one at a time until each orbital of a sublevel contains 1e- each, spinning in the same direction, and then they begin to double up. When they double up the electrons spin in opposite directions. ↑ ↑ px ↑↓ Px Px ↑ ↑ y z ↑ ↑ ↑ y z ↑↓ ↑↓ y z Px y ↑↓ ↑↓ ↑↓ ↑ Px ↑ y z Px 6 Electron Configurations of the Elements #1 - 4 1 H orbital notation 1s1 electron configuration 2 He 1s2 3 Li 1s2 2s1 1s2 2s2 4 Be 7 Physics 2000 Link TOC → Elements as atoms → Beyond Hydrogen → Periodic Table Dave's Whizzy Periodic Table 8 Electron Configurations of the Elements #6 - 10 5 B 1s2 2s2 2p x1 y0 z0 1s2 2s2 2p x1 y1 z0 1s2 2s2 2p x1 y1 z1 1s2 2s2 2p x2 y1 z1 1s2 2s2 2p x2 y2 z1 1s2 2s2 2p x2 y2 z2 6 C 7 N 8 O 9 F 10 Ne 9 Complete the following: 11 Na 1s2 2s2 2p x2 y2 z2 3s1 1s2 2s2 2p x2 y2 z2 3s2 1s2 2s2 2p x2 y2 z2 3s2 3p x1 y0 z0 1s2 2s2 2p x2 y2 z2 3s2 3p x1 y1 z0 1s2 2s2 2p x2 y2 z2 3s2 3p x1 y1 z1 1s2 2s2 2p x2 y2 z2 3s2 3p x2 y1 z1 1s2 2s2 2p x2 y2 z2 3s2 3p x2 y2 z1 1s2 2s2 x2 z2 3s2 3p x2 y2 z2 12 Mg 13 Al 14 Si 15 P 16 S 17 Cl 18 Ar 2p y2 10 Ar is a noble gas. The 3rd energy level is its outermost and it has a stable octet with 2e- in 3s and 6e- in 3p. )1 )2 )3 )4 s sp spd spdf Electrons fill an atom from the nucleus out. Energy levels are NOT equally spaced. As you get further from the nucleus, energy levels get closer together and overlap. Aufbau Principle: electrons enter orbitals of Ex.: lowest energy first 4s fills before 3d, so 4s is of lower energy than the 3d sublevel, even though the 4s orbital is part of a higher energy level! Note: After the 3p sublevel is filled, the 4s sublevel begins to fill because this is where the overlap of energy levels begins! *Energy levels are NOT evenly spaced. As you go further out from the nucleus, the energy levels get closer together and overlap more and more! 11 Because electrons “want” to be in the orbital with the LOWEST energy, (remember the AUFBAU PRINCIPLE), there are some EXCEPTIONS to how sublevels fill up: Sublevel Energy Highest partly full ½ full full Lowest Chromium (Cr) empty 4s12 Molybdenium (Mo) 3d54 5s12 Copper (Cu) 4d54 4s12 Silver (Ag) 9 3d10 5s12 9 4d10 12 Order of Filling Guide 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 6f* 7s 7p* 7d* 7f* *Although these sublevels exist in theory, there is no practical use for them, since all naturally occurring, stable elements are accounted for up through 6d. 13 Bonding: When elements bond (combine to form compounds), only the electrons in Lewis Dot Diagrams the outer shell (energy level) are involved. Chemists use _____________________ to show these outer shell electrons. Lewis Dot Diagrams: Take into account the electrons from the highest energy levels’ s and p sublevels. Write the symbol for the element and use dots to represent electrons. Place the dots one on each side before you pair them up (just like filling orbitals). Ex.: H 1s1 H (1) Be C 1s2 2s2 1s2 2s2 2p2 Be C (2) Bonding electrons (4) (2) S Ne 1s2 2s2 2p6 3s2 3p4 S 1s2 2s2 2p6 Ne Paired electrons do NOT bond NO bonding electrons (STABLE OCTET) 14 All TRANSITION metals and INNER TRANSITION metals have a completed s sublevel, so their Lewis Dot Diagrams all look the same: Ex.: Fe 1s2 2s2 2p6 3s2 3p6 4s2 3d6 Fe Pm 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f4 Pm 15 Use of the Periodic Table to Write Electron Configurations for Elements d block – group “B” transition metals; middle of table (10e-) p block – last 6 columns on table (6e-) f block – bottom block on table; “inner transition” metals (14e-) s block – 1st & 2nd column on table (2e-) (Designate energy level overlaps on your periodic chart) 16 Use your periodic chart (or the Order of Filling Chart) to write Electron Configurations for each of the following: Abbreviated Form: Ni # 28 1s2 2s2 2p6 3s2 3p6 4s2 3d8 [Ar] 4s2 3d8 I # 53 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5 [Kr] 5s2 4d10 5p5 Au # 79 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d9 [Xe] 6s2 4f14 5d9 2n2 rule: (use “n” to indicate the number of an energy level) n n This rule is used to determine the number of sublevels, number of orbitals, or number of total electrons in ANY GIVEN ENERGY LEVEL! How many sublevels, orbitals = energy level # [row of periodic table] & e- could be contained in the 8th energy level? = # of sublevels n2 = # of orbitals n = 8 (= # energy levels & # sublevels) 2n2 = # of electrons n2 = 64 orbitals 2n2 = 128 e- total 17 Atomic orbitals - orbital of an atom, a 3-D region of space with a high probability of finding an e- (fly in a jar). spherical s orbitals are _______________ 2-lobed x, y, and z axes p orbitals are _______________ and oriented around the ____________________ 4-lobed d orbitals are _______________ with each lobe located ____________________ in one of the quadrants of the x, y, and z axes __________________________________________________________________ 5-lobed p or d orbitals f orbitals are _______________ and more complex than ____________________ (f orbitals do not get involved ion bonding) ORBITAL SHAPES s orbital p orbital 18 d orbital Note: 1. At higher energy levels, the lobes are the same shape, but farther from the nucleus. 2. When all orbitals of a sublevel are filled, the resulting shape is spherical. 19 Light and the Quantum Mechanical Model Light Is made up of Waves 20 WAVES Amplitude Wave’s height from zero to the crest. Wavelength λ Frequency ν Distance between the crests; crest to crest. Unit meters (m) Number of wave cycles to pass A given point per unit of time. Unit is the Hertz (Hz) 21 The wavelength and frequency of light are Inversely proportional to each other. As the wavelength of light increases, the frequency decreases and vice versa. 22 Atomic Spectra Ground State Lowest possible energy of an electron When electrons absorb energy they can jump from ground state to an excited state (a higher energy level). When the electron drops down to a lower energy level a quantum (bundle) of energy is released in the form of light. Each quantum produces a specific amount of energy that corresponds to the lines shown on an atomic spectrum. Each element has its own unique atomic spectrum. 23