holtchp14review - Marshall Middle School
advertisement

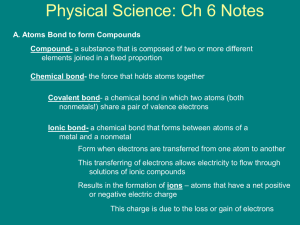
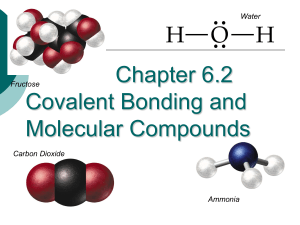
Holt Chapter 14 Chapter Review Using Vocabulary • 1. The force of attraction that holds two atoms together is a chemical bond. • 2. Charged particles that form when atoms transfer electrons are ions. • 3. The force of attraction between the nuclei of atoms and shared electrons is a covalent bond. • 4. Electrons free to move throughout a material are associated with a metallic bond. • Shared electrons are associated with a covalent bond. Understanding Concepts • 6. Which element has a full outermost energy level containing only 2 electrons? • a) oxygen b) hydrogen c) fluorine • d) helium • Answer: d) Helium • 7. Which (of the following) describes what happens when an atom becomes an ion with a 2- charge? • Answer: c) the atom gains 2 electrons • 8. The properties of ductility and malleability are associated with which type of bonds? • a) ionic b) covalent c) metallic d) none of the above • Answer: c) metallic • 9. In which area of the periodic table do you find elements whose atoms easily gain electrons? • Answer: c) on the right side • 10. What type of element tends to lose electrons when it forms bonds? • A) metal b) metalloid c) nonmetal d) noble gas • Answer: a) metals • 11. Which pair of atoms can form an ionic bond? • Answer: • b) potassium (K) and fluorine (F) Short Answers • 12. List two properties of covalent compounds. • Answers could include: • - a low melting point, • - a low boiling point, • - brittle when solid at room temp. • 13. Explain why an iron ion is attracted to a sulfide ion but NOT to a zinc ion? • Answer: iron and zinc are metals that tend to lose electrons , thus becoming positively charged. They would repel each other. • Sulfide ion is negatively charge sulfur atom that gained electron, so is attracted to a positively charge atom such as iron • 14. Using knowledge of valence electrons, explain why carbon is an element in so many different molecules. • Carbon has 4 valence electrons, therefore can have 4 bonds, including other carbon atoms. This is the most bonding possibilities. Usually atoms with less than 4 valence electrons give up electrons, and may only have one bond. • 15. Three types of bonds: • Ionic - lose/gain electrons • Covalent - share electrons • Metallic - electrons can move between ions within a metal Concept map • 16. This was put on the board Critical Thinking • 17. Types of bonding of these pairs: • • • • A) zinc + zinc = metallic B) oxygen + nitrogen = covalent C) phosphorus and oxygen = covalent D) magnesium and chlorine = ionic • 18. Dots & bonds • • • • • A) sulfur (S); 6 dots, 2 bonds B) nitrogen (N); 5 dots, 3 C) neon (Ne); 8 dots, 0 bonds D) iodine (I); 7 dots, 1 bond E) silicon (Si); 4 dots, 4 bonds • 19. What type of bond does the picture of a hammer is hitting a white, solid object with little parts appearing on the table represent.? • Answer: The substance is brittle, and is breaking into tiny pieces, therefore is an ionic bond. Math in Science • • • • • • 20.Electrons gained/loss and charge Answers: A) calcium (Ca); lose 2 e-; 2 + B) phosphorus (P); gain 3e-; 3 C) bromine (Br); gain 1e-; 1D) sulfur (S); gain 2e-; 2 - Interpreting Graphics • 21. The metal band near the eraser is the part of the pencil where metallic bonds are formed. • 22. Molecules with covalent bonds in a pencil include: • - graphite • - wood • - eraser • 23. Two differences between the properties of the parts of the pencil are: • Answer: The metallically bonded part of the pencil is shiny, can be bent without breaking, and is hard in texture; • The covalently bonded parts are not shiny, will break when bent, and are softer. Example: eraser, graphite, wood • The End