PowerPoint for Chapter 6 Part I
advertisement

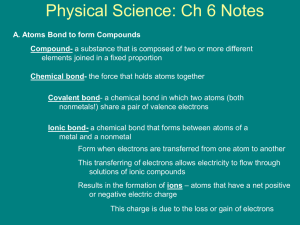
Chemical Bonding Chapter 6 Pages 174-213 (no Section 4) The breaking of bonds and the forming of bonds occur during chemical reactions. Aspirin What is the formula for a molecule of aspirin? Is it an ionic or covalent (molecular) compound? What do we call the things that hold a molecule of aspirin together? • C9H8O4 • covalent compound (made of all nonmetals - no ions) Aspirin • C9H8O4 • covalent compound (made of all nonmetals - no ions) Aspirin (Odyssey Program) • C9H8O4 • covalent compound (made of all nonmetals - no ions) The Attachment Between Atoms atoms combine to form ionic bonds (M + NM) covalent bonds (NM + NM) chemical bond – a mutual electrical attraction between the nuclei and valence electrons of two atoms that binds the atoms together Ionic Bonding • ionic bond – electrical attraction between cations and anions; when electrons are taken by one atom from another atom metal and a nonmetal NaCl cation and anion (The charges are “hidden” to make a neutral compound.) Ionic Bonding: taking of electrons Na 11e- F 9e- Na+ 10e- STABLE!!! F- 10e- STABLE!!! 3s 3s 2p 2p 2s 2s 1s 1s I’m Positive! A metal ion atom A nonmetal ion atom I’m Negative I’m Positive! A metal ion A nonmetal ion I’m Negative When a metal and a nonmetal atom are around each other there is the opportunity for…. …the transfer of electrons producing ions that would like to cling to each other. Ionic bonding!!! The simplest ratio of the packed ions is called: “cubic” shape The Formula Unit Ex: NaCl Ions • Metals form cations. (metals lose e ) • Nonmetals form anions. (nonmetals gain e ) Ions • cations (+) • anions (-) • monatomic ions – ions • formed from one atom Na+ or O-2 Examples: • polyatomic ions - ions formed from two or more atoms bonded together Examples: NH4+ or SO4-2 Naming Ions • monatomic ions • cations – named like the atom, only add ion to it » Example: Na+ is the sodium ion • anions – remove the ending to the atom name and add –ide and ion to it » Example: Clis the chlorine +ide ion or the chloride ion • polyatomic ions • You do not determine their names, you memorize them Ionic Compounds • solid at room temperature (forming crystals) • high melting points (thus are usually solid at RT) • formula unit represents the lowest ratio of ions that combine to form a neutral compound • when dissolved in water, the ionic compounds will break up into ions (dissociate) • the solutions of ionic compounds will conduct electricity (electrolytes) Dissociation NaCl(s) Na (aq) + Cl (aq) H2O(l) solid placed in water + - hydrated ions (surrounded by water) dissociation – when an ionic compound dissolves to break apart into hydrated ions Dissociation Electrolytes and Nonelectrolytes When an ionic compound dissolves to produce ions, it is called an electrolyte because it conducts electricity in water. When an compound does not dissolve to produce ions, it is called a nonelectrolyte because it does not conduct electricity in water. Electrolytes or salt? Check for Understanding 1. What kinds of atoms form ionic bonds? 2. What is a polyatomic ion? 3. Name 5 things you learned about ionic compounds. You Try It. Do the Dissociation Equations worksheet. Covalent Bonding covalent bond – when electrons are shared between two atoms – the electronegativity difference between the two atoms is less than 1.7 – usually two nonmetals – NO ions formed! (no electrons are taken…just shared) When a nonmetal and another nonmetal atom are around each other there is the opportunity for…. …the sharing of electrons producing molecules in which the atoms like to cling to each other. Covalent bonding!!! The formation of a bond between two nonmetal atoms. Atoms sufficiently far apart to have no interaction Figure 5 Page 179 Covalent Compounds • • • • Also called molecular compounds solid, liquid, or gas at room temperature low melting points molecular formula represents the actual ratio of atoms that combine to form a neutral compound • when dissolved in water, the molecular compounds DO NOT break up into ions (NO dissociation) • the solutions of molecular compounds DO NOT conduct electricity (nonelectrolytes) Pure Covalent The two fundamental types of bonds. Ionic There is another type of bond, not purely covalent and not purely ionic. Nonpolar Pure Covalent Polar Covalent Ionic Sharing of Electrons • How would you know if an electron is going to be taken by one atom from another? • Is there ever a time in which the electron is not taken but shared? • Is the electron always shared equally? Electronegativity • electronegativity – a measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound The difference in electronegativity values for two atoms will indicate whether the two atoms form an ionic bond (e- taken) or a polar or nonpolar covalent bond (e- shared). Electronegativity Differences • • • 0.0 to 0.4 nonpolar covalent 0.5 to 1.6 polar covalent 1.7 and up ionic These ranges are flexible, although the general rule is a metal and nonmetal will form an ionic bond and two nonmetals will form a covalent bond. (Learn these values!) PS: They are different than your book! Ionic, Polar Covalent, or Nonpolar Covalent? What kind of bond would each pair form? 1. N and S 2. S and C 3. Mg and Cl 4. C and F 5. Ba and O Which one of these bonds has the least ionic character? Valence Electrons • valence electrons – the electrons in the highest energy level Na: 1s22s22p63s1 - 1 valence eO: ? Ne: ? Al: ? He: ? Octet Rule • octet rule – most atoms will gain or lose electrons to have 8 valence electrons (e- in the highest energy level) – Exceptions: H, He, Li, Be, B, and some atoms P and higher on the periodic table Why is an atom like Ca more stable once it becomes an ion? How many valence electrons would calcium have to lose to have 8? VSEPR Theory Valence Shell Electron Pair Repulsion Repulsion between the sets of valence-level electrons surrounding an atom causes these sets to be oriented as far apart as possible. Theory Regions of Electron Density What is a Region of electron density? • Single bond (2e- connecting 2 atoms) • Double bond (4e- connecting 2 atoms) • Triple bond (6e- connecting 2 atoms) • Lone pair (unbonded pair) (2e- alone on an atom) LINEAR 180o 2 Regions of Electron Density 2 Bonds bonded pair of electrons bonded pair of electrons TRIGONAL PLANAR 120o 3 Regions of Electron Density 3 Bonds 3 bonded pairs of electrons You don’t have to know this! BENT 119o 3 Regions of Electron Density 2 Bonds & 1 Lone Pair 2 bonded pairs of electrons 1 lone pair of electrons TETRAHEDRAL 109.5o 4 Regions of Electron Density 4 Bonds 4 bonded pairs of electrons TRIGONAL PYRAMIDAL 107o 4 Regions of Electron Density 3 Bonds & 1 Lone Pair 1 lone pair of electrons 3 bonded pairs of electrons BENT 105o 4 Regions of Electron Density 2 Bonds & 2 Lone Pairs All of these have 4 regions of electron density (although the number of bonded pairs is different) TRIGONAL BIPYRAMIDAL 120o & 90o 5 Regions of Electron Density 5 Bonds SEE-SAW You don’t have to know this! 120o & 90o 5 Regions of Electron Density 4 Bonds & 1 Lone Pair T-SHAPED You don’t have to know this! 120o & 90o 5 Regions of Electron Density 3 Bonds & 2 Lone Pairs LINEAR You don’t have to know this! 180o 5 Regions of Electron Density 2 Bonds & 3 Lone Pairs OCTAHEDRAL 90o 6 Regions of Electron Density 6 Bonds SF6 SQUARE PYRAMIDAL approximately 90o 6 Regions of Electron Density 5 Bonds & 1 Lone Pair BrF5 You don’t have to know this! SQUARE PLANAR 90o 6 Regions of Electron Density 4 Bonds & 2 Lone Pairs ICl4-