Ionic bonding lesson objective:
advertisement
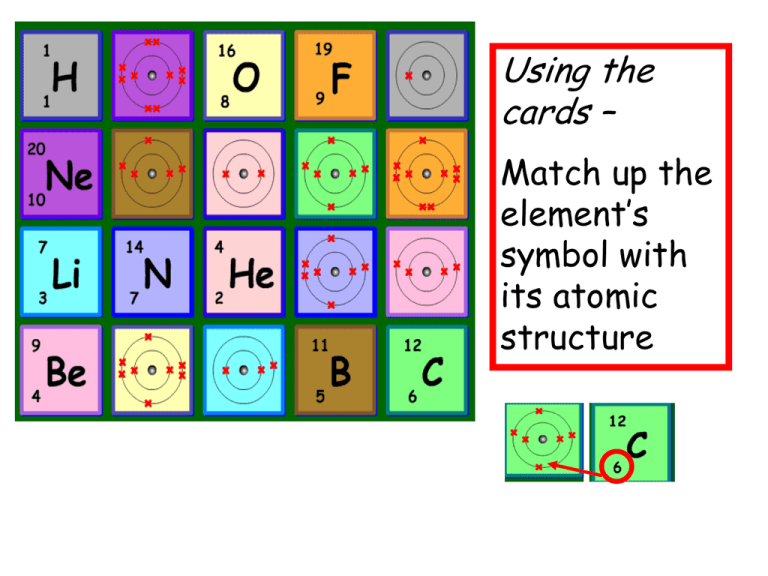
Using the cards – Match up the element’s symbol with its atomic structure Ionic bonding lesson objective: To understand how ionic bonding occurs Btec -Working towards pass merit distinction between atoms Lesson outcomes: 1. Draw dot and cross diagrams to show ionic bonding 2. Describe the properties of an ionic compound. 3. Explain how an ionic bond forms. Bonding basics Complete the chart using your atomic structure cards Element Lithium Number of electrons 3 Beryllium 1 Group number 1 2 5 8 Fluorine Number of electrons on outside shell 3 6 Easy to lose or gains and an electron? Bonding basics Complete the chart using your atomic structure cards Element Lithium Beryllium Number of electrons 3 Number of electrons on outside shell 1 Group number Easy to lose or gains and an electron? 1 lose 2 lose 3 3 lose atom 2 gains 4Whenoranloses an Electron it is called an Ion Boron 5 Oxygen 8 6 6 Gain Fluorine 9 7 7 Gain Ionic Bonds • Ionic bonds are formed by one atom transferring electrons to another atom to form ions. • Ions are atoms, which have lost or gained electrons and therefore have a negative or positive charge • The atom losing electrons forms a positive ion and is usually a metal. Sodium atom + + + + + + + + + + + - • The atom gaining electrons forms a negative ion and is usually a non-metallic element. + + + + + + + + + + - + + + + + + + + - XXXX X X XX X X X X X X X XX Chlorine atom Sodium atom Chlorine atom Electron transfer XXXX X X XX X X X X X + - Loses electron Gains electron Positive and negative ions attract X X XX Ionic bonding Sodium Chloride XXXX X X XX - + Positive and negative ions attract X X X X X X X XX Answer these questions: NEGATIVE An atom that gains one or more electrons will have a ____________________ charge. POSITIVE An atom that loses one or more electrons will have a ____________________ charge. ION An atom that gains or loses one or more electrons is called an ____________. What is an ionic bond? ELECTRONS to another to form the bond. Atoms will transfer one or more ________________ Each atom is left with a ________________ COMPLETE outer shell. An ionic bond forms between a ___________ METAL ion with a positive charge and a ________________ NONMETAL ion with a negative charge attracting each other. Example: Sodium + Chlorine XXXX X X XX Sodium chloride X X X X X X X XX Games for learning – dominoes Which domino below will match Chlorine? Sodium chloride Which domino below will match oxygen? Sodium chloride Lithium oxide Groups 1 Groups 2 3 4 5 6 7 0 The periodic table for ionic bonding Metals Non metals What's wrong? Li Ca Li Li Ca Li Li Li Li Li S Mg Mg S Mg Ca Mg Ca S S Ionic bonding lesson objective: To understand how ionic bonding occurs Btec -Working towards pass between atoms Lesson outcomes: Draw dot and cross diagrams to show ionic bonding Ionic bonding lesson objective: To understand how ionic bonding occurs Btec -Working towards merit between atoms Lesson outcomes: Draw dot and cross diagrams to show ionic bonding and describe the properties of an ionic compound. Ionic bonding lesson objective: To understand how ionic bonding occurs Btec -Working towards between atoms Lesson outcomes: Draw dot and cross diagrams to show ionic bonding and describe the properties of an ionic compound. distinction Explain how an ionic bond forms. Metal Non Metal Compound Ion