sy26_apr22_10
advertisement

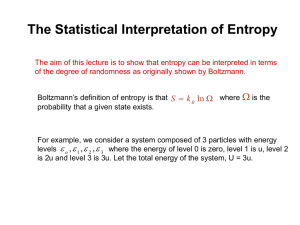
Lecture 25 Goals: • Chapter 18 Understand the molecular basis for pressure and the idealgas law. Predict the molar specific heats of gases and solids. Understand how heat is transferred via molecular collisions and how thermally interacting systems reach equilibrium. Obtain a qualitative understanding of entropy, the 2nd law of thermodynamics • Assignment HW12, Due Tuesday, May 4th For this Tuesday, Read through all of Chapter 19 Physics 207: Lecture 26, Pg 1 Macro-micro connection Mean Free Path If a molecule, with radius r, averages n collisions as it travels distance L, then the average distance between collisions is L/n, and is called the mean free path λ The mean free path is independent of temperature The mean time between collisions is temperature dependent Physics 207: Lecture 26, Pg 2 The mean free path is… Some typical numbers Vacuum Pressure (Pa) Molecules / cm3 Molecules/ m3 mean free path Ambient pressure Medium vacuum 105 2.7*1019 2.7*1025 68 x 10-9 m 100-10-1 1016 – 1013 1022-1019 Ultra High vacuum 10-5-10-10 109 – 104 0.1 - 100 mm 1015 – 1011 1-105 km Physics 207: Lecture 26, Pg 3 Distribution of Molecular Speeds A “Maxwell-Boltzmann” Distribution O2 at 25°C 1.4 # Molecules 1.2 1.0 O2 at 1000°C 0.8 Fermi Chopper 1 0.6 2 0.4 0 1 Most probable 2 Mean (Average) 200 600 1000 1400 1800 Molecular Speed (m/s) Physics 207: Lecture 26, Pg 4 Macro-micro connection Assumptions for ideal gas: # of molecules N is large They obey Newton’s laws Short-range interactions with elastic collisions Elastic collisions with walls (an impulse…..pressure) What we call temperature T is a direct measure of the average translational kinetic energy What we call pressure p is a direct measure of the number density of molecules, and how fast they are moving (vrms) Relationship between Average Energy per Molecule & Temperature Physics 207: Lecture 26, Pg 5 Macro-micro connection One new relationship 3 k BT avg 2 2 T avg 3k B 2N p avg 3V vrm s (v ) avg 2 3k BT m Physics 207: Lecture 26, Pg 6 Exercise Consider a fixed volume of ideal gas. When N or T is doubled the pressure increases by a factor of 2. 1 2 3 mv kB T 2 2 pV N kBT 1. If T is doubled, what happens to the rate at which a single molecule in the gas has a wall bounce (i.e., how does v vary)? (A) x1.4 (B) x2 (C) x4 2. If N is doubled, what happens to the rate at which a single molecule in the gas has a wall bounce? (A) x1 (B) x1.4 (C) x2 Physics 207: Lecture 26, Pg 8 A macroscopic “example” of the equipartition theorem Imagine a cylinder with a piston held in place by a spring. Inside the piston is an ideal gas a 0 K. What is the pressure? What is the volume? Let Uspring=0 (at equilibrium distance) What will happen if I have thermal energy transfer? The gas will expand (pV = nRT) The gas will do work on the spring +Q Conservation of energy Q = ½ k x2 + 3/2 n R T (spring & gas) and Newton S Fpiston= 0 = pA – kx kx =pA Q = ½ (pA) x + 3/2 n RT Q = ½ p V + 3/2 n RT (but pV = nRT) Q = ½ nRT + 3/2 n RT (25% of Q went to the spring) ½ nRT per “degree of freedom” Physics 207: Lecture 26, Pg 9 Degrees of freedom or “modes” Degrees of freedom or “modes of energy storage in the system” can be: Translational for a monoatomic gas (translation along x, y, z axes, energy stored is only kinetic) NO potential energy Rotational for a diatomic gas (rotation about x, y, z axes, energy stored is only kinetic) Vibrational for a diatomic gas (two atoms joined by a spring-like molecular bond vibrate back and forth, both potential and kinetic energy are stored in this vibration) In a solid, each atom has microscopic translational kinetic energy and microscopic potential energy along all three axes. Physics 207: Lecture 26, Pg 10 Degrees of freedom or “modes” A monoatomic gas only has 3 degrees of freedom (x, y, z to give K, kinetic) A typical diatomic gas has 5 accessible degrees of freedom at room temperature, 3 translational (K) and 2 rotational (K) At high temperatures there are two more, vibrational with K and U to give 7 total A monomolecular solid has 6 degrees of freedom 3 translational (K), 3 vibrational (U) Physics 207: Lecture 26, Pg 11 The Equipartition Theorem The equipartition theorem tells us how collisions distribute the energy in the system. Energy is stored equally in each degree of freedom of the system. The thermal energy of each degree of freedom is: Eth = ½ NkBT = ½ nRT A monoatomic gas has 3 degrees of freedom 5 A diatomic gas has 5 degrees of freedom E nRT th 2 A solid has 6 degrees of freedom E 3nRT th 3 Eth nRT 2 Molar specific heats can be predicted from the thermal energy, because Eth nCT Monoatomic gas Diatomic gas Elemental solid 3 CV nRT 2 CV 3nRT 5 CV nRT 2 Physics 207: Lecture 26, Pg 12 Exercise A gas at temperature T is an equal mixture of hydrogen and helium gas. Which atoms have more KE (on average)? (A) H (B) He (C) Both have same KE How many degrees of freedom in a 1D simple harmonic oscillator? (A) 1 (B) 2 (C) 3 (D) 4 (E) Some other number Physics 207: Lecture 26, Pg 13 The need for something else: Entropy V1 You have an ideal gas in a box of volume V1. Suddenly you remove the partition and the gas now occupies a larger volume V2. P (1) How much work was done by the system? P (2) What is the final temperature (T2)? V2 (3) Can the partition be reinstalled with all of the gas molecules back in V1? Physics 207: Lecture 26, Pg 14 Free Expansion and Entropy V1 You have an ideal gas in a box of volume V1. Suddenly you remove the partition and the gas now occupies a larger volume V2. P (3) Can the partition be reinstalled with all of the gas molecules back in V1 P V2 (4) What is the minimum process necessary to put it back? Physics 207: Lecture 26, Pg 17 Free Expansion and Entropy V1 You have an ideal gas in a box of volume V1. Suddenly you remove the partition and the gas now occupies a larger volume V2. P (4) What is the minimum energy process necessary to put it back? P Example processes: V2 A. Adiabatic Compression followed by Thermal Energy Transfer B. Cooling to 0 K, Compression, Heating back to original T Physics 207: Lecture 26, Pg 18 Exercises Free Expansion and the 2nd Law What is the minimum energy process necessary to put it back? V1 P Try: B. Cooling to 0 K, Compression, Heating back to original T Q1 = n Cv T out and put it where…??? P V2 Need to store it in a low T reservoir and 0 K doesn’t exist Need to extract it later…from where??? Key point: Where Q goes & where it comes from are important as well. Physics 207: Lecture 26, Pg 19 Modeling entropy I have a two boxes. One with fifty pennies. The other has none. I flip each penny and, if the coin toss yields heads it stays put. If the toss is “tails” the penny moves to the next box. On average how many pennies will move to the empty box? Physics 207: Lecture 26, Pg 20 Modeling entropy I have a two boxes, with 25 pennies in each. I flip each penny and, if the coin toss yields heads it stays put. If the toss is “tails” the penny moves to the next box. On average how many pennies will move to the other box? What are the chances that all of the pennies will wind up in one box? Physics 207: Lecture 26, Pg 21 2nd Law of Thermodynamics Second law: “The entropy of an isolated system never decreases. It can only increase, or, in equilibrium, remain constant.” Increasing Entropy Entropy measures the probability that a macroscopic state will occur or, equivalently, it measures the amount of disorder in a system The 2nd Law tells us how collisions move a system toward equilibrium. Order turns into disorder and randomness. With time thermal energy will always transfer from the hotter to the colder system, never from colder to hotter. The laws of probability dictate that a system will evolve towards the most probable and most random macroscopic state Physics 207: Lecture 26, Pg 22 Entropy Two identical boxes each contain 1,000,000 molecules. In box A, 750,000 molecules happen to be in the left half of the box while 250,000 are in the right half. In box B, 499,900 molecules happen to be in the left half of the box while 500,100 are in the right half. At this instant of time: The entropy of box A is larger than the entropy of box B. The entropy of box A is equal to the entropy of box B. The entropy of box A is smaller than the entropy of box B. Physics 207: Lecture 26, Pg 23 Entropy Two identical boxes each contain 1,000,000 molecules. In box A, 750,000 molecules happen to be in the left half of the box while 250,000 are in the right half. In box B, 499,900 molecules happen to be in the left half of the box while 500,100 are in the right half. At this instant of time: The entropy of box A is larger than the entropy of box B. The entropy of box A is equal to the entropy of box B. The entropy of box A is smaller than the entropy of box B. Physics 207: Lecture 26, Pg 24 Reversible vs Irreversible The following conditions should be met to make a process perfectly reversible: 1. Any mechanical interactions taking place in the process should be frictionless. 2. Any thermal interactions taking place in the process should occur across infinitesimal temperature or pressure gradients (i.e. the system should always be close to equilibrium.) Based on the above answers, which of the following processes are not reversible? 1. Melting of ice in an insulated (adiabatic) ice-water mixture at 0°C. 2. Lowering a frictionless piston in a cylinder by placing a bag of sand on top of the piston. 3. Lifting the piston described in the previous statement by slowly removing one molecule at a time. 4. Freezing water originally at 5°C. Physics 207: Lecture 26, Pg 25 Reversible vs Irreversible The following conditions should be met to make a process perfectly reversible: 1. Any mechanical interactions taking place in the process should be frictionless. 2. Any thermal interactions taking place in the process should occur across infinitesimal temperature or pressure gradients (i.e. the system should always be close to equilibrium.) Based on the above answers, which of the following processes are not reversible? 1. Melting of ice in an insulated (adiabatic) ice-water mixture at 0°C. 2. Lowering a frictionless piston in a cylinder by placing a bag of sand on top of the piston. 3. Lifting the piston described in the previous statement by removing one grain of sand at a time. 4. Freezing water originally at 5°C. Physics 207: Lecture 26, Pg 26 Lecture 26 • To recap: HW12, Due Tuesday May 4th For this Tuesday, read through all of Chapter 19! Physics 207: Lecture 26, Pg 30