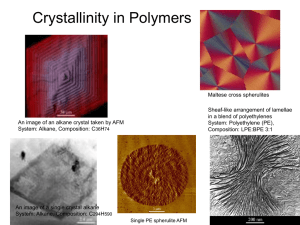
Crystallinity in polymers: an
historical view
Geoffrey Allen"
We still have much to learn about the morphologies of
natural and synthetic polymers crystallized in bulk.
Nevertheless, conventional X-ray and thermodynamic
studies of crystallinity in polymeric materials over the past
60 years have given us a clear picture of the concept of
crystallinity and the molecular features which promote
crystallinity in these substances. Apart from issues of chain
connectivity, the science of conventional molecules proved
adequate for the task!
A few liquids of low molecular weight do not
readily crystallize. Apparently these substances are
composed of molecules that have some difficulty
in arranging themselves into a crystalline array.
The molecules usually have bulky substituent
groups which offer steric resistance to folding into
a compact form suitable for efficient packing in a
crystal lattice. Such liquids, on cooling, become
very viscous and ultimately form glasses at
temperatures around — 100°C. In this solid state
they show no sharp X-ray diffraction rings
indicative of randomly orientated crystals. In fact,
the diffuse X-ray diffraction pattern observed is
very similar to that of the corresponding liquid,
showing that the glassy solid has no long-range
molecular order. Some of these liquids can be
crystallized by holding a sample for many hours at
a specific temperature, some 20°C above the glass
formation temperature, until the substance suddenly
begins to crystallize. On warming, the melting point
of the crystals is well defined and located about
40—60°C above the temperature of glass formation.
Given that some relatively simple small molecules
find difficulty in forming crystals, what are we to
expect of the behaviour of polymer molecules?
A typical simple polymer molecule has some
10000 repeat units covalently linked into a long
chain, for example:
polyethylene-
-(CH 2 -CH 2 HCH 2 -CH 2 HCH 2 -CH 2 HCH 2 -CH 2 )-
polyethyleneoxide
-(CH 2 -CH 2 -OHCH 2 -CH 2 -O)-
and the chains may not be linear, as is often the case in polyethylene: for example
—CH2— CH2— CH2— CH—CH2—CH2—CH2—CH2—CH—CH2—CH2—
CH 2
CH2
CH 2
CH2
CH3
CH2
I
I
CH2
"Kobe Steel Ltd., Alton House, 174/177 High Holborn,
London WC1V 7AA, UK.
1062-7987/93/020197-10 $10.00
© 1993 by John Wiley & Sons, Ltd.
CH3
EUROPEAN REVIEW, Vol. 1, No. 2, 197-206 (1993)
Geoffrey Allen
198
since molecular branches can be formed during the polymerization process.
Even the linear chains of larger monomer units will have sizeable side groups: for example
polystyrene
CeHs
C9H5
C6H5
I
I
I
-CH 2 -CH-CH 2 -CH-CH 2 -CHCH3
CH3
CH3
I
I
I
polypropylene -CH 2 -CH-CH 2 -CH-CH 2 -CHR
R
R
I
polypeptide
I
I
-CH-C-NH-CH-C-NH-CH-C-NH-
II
O
and R may represent several different side groups
in the same polypeptide chain.
Now, bear in mind that each molecule can curl
into a randomly arranged coil, by virtue of
rotations about the bonds in the main chain. The
polymer melt is composed of these randomly
coiled chains and each chain is wriggling rapidly
through millions of conformations. Furthermore,
the long chains are entangled. To fit into a crystal
lattice, a chain must uncoil a well-defined section
of say 10—20 monomer units and align that section
with a similar one from another part of the same
chain or of a neighbouring chain. If a sufficient
number of segments can line up to form a stable
nucleus then crystal growth can occur. A priori, it
would seem to be an event of low probability even
in polymers composed of linear long chains of the
simplest chemical structure!
In fact, crystallization offlexiblechain molecules
of sufficient structural regularity is widely observed
in naturally occurring polymer systems such as
polypeptides, carbohydrates and natural rubber, as
well as in those of synthetic origin. The first
recorded observation was made by John Gough1
in a letter to the Manchester Literary and
Philosophical Society in 1805:
' If a thong of Caoutchouc be stretched, in water
warmer than itself, it retains its elasticity
unimpaired. If the experiment be made in water
cooler than itself, it loses part of its retractile
power, being unable to recover its former figure;
but let the thong be placed in hot water, while
it remains extended for want of spring, and the
heat will immediately make it contract briskly'.
Gough did not realize he was witnessing the partial
II
O
II
O
crystallization of the rubber strip followed by its
melting in the warmer water.
The systematic study of crystalline polymers
parallels the development of polymer science from
its origins in the early 1920s. Following the
discovery that crystalline substances diffract X-rays
and that the diffraction pattern can be resolved to
give unit cells and hence crystal structures, Michael
Polanyi (in his days as a physical chemist, before
he became a professional philosopher) obtained
diffraction patterns from a bundle of parallel ramie
fibres. Polanyi2 concluded that the pattern of spots
corresponded to crystallites of cellulose orientated
with one axis parallel to the fibre axis. The
empirical structure of cellulose was known to be
C 6 H 10 O 5 ; Polanyi found that the unit cell was
orthorhombic, measuring 7.9 x 8.4 x 10.2 A (1 A =
10~ lo m). He concluded that the cell contained
either two cyclic disaccharides or two disaccharide
units of polysaccharide chains. The concept of the
existence of polymer chains was not at the time
accepted by organic chemists and the second
option was not well received. Polanyi3 wrote in his
memoirs in 1962:
'Unfortunately I lacked the chemical sense for
eliminating the first alternative . . .
I was gleefully witnessing the chemists at cross
purposes with conceptual reform . . .
I should have been better occupied in definitely
establishing the chain structure.'
In the mid 1920s, Katz4 observed that an
amorphous natural rubber strip when extended
six-fold produces a sharp X-ray diffraction pattern
' characteristic of a body containing crystallites' in
addition to the amorphous halo. Katz estimated
Crystallinity in polymers
that the same cell could only accommodate three
isoprene units. In the same year, 1925, Staudinger
and his colleagues5 published studies of 'oligomers
and high polymers' (that is polymer chains so long
that the molecular weight could not be determined
by the methods then available) of formaldehyde,
• • -O-CH 2 -O-CH 2 -O-CH 2 -O-CH 2 -- • •
X-ray diffraction from the oligomers showed
reflections characteristic of the length of the
extended molecules but in addition there were
reflections due to a subcell that enclosed sections
of the chain. As the length of the oligomers
increased, the subcell reflections became more
pronounced and it was possible to retain by
extrapolation the diffraction pattern of the high
polymer. By 1928, X-ray studies by K. H. Meyer
and Herman Mark6 demonstrated that the crystal
structures of cellulose, silk fibroin, chitin and
natural rubber were due to the packing of
long-chain molecules. The concept of a long-chain
molecule was fully accepted.
The paper on silk fibroin described for the first
time a polypeptide chain in the fully extended
conformation—now known as the ^-structure.
In the next decade Astbury7 found that there were
two crystalline forms, a and /?. Later, from
crystallographic measurements on aminoacids and
peptides Pauling and his school argued that the
/?-form was composed of helical molecules with 3.6
amino acids per turn and a pitch of 1.5 A. Pauling8
deduced this structure from molecular models as
well as crystallographic data. Later still, this
combination was to be crucial in the work of Crick
and Watson9 on the structure of DNA. The X-ray
diffraction method is now a cornerstone of
molecular biology.
Many common synthetic polymers such as
polystyrene and polymethylmethacrylate (Perspex)
did not crystallize, they were only available as
glassy (amorphous) substances in the solid state.
Furthermore, the various known forms of
polyethylene crystallized only to a degree of about
40%, that is, they remained a mixture of crystalline
and amorphous fractions. However, in the early
1950s Ziegler10 reported a new family of catalysts
which produced highly crystalline polyethylene of
high molecular weight. Natta11 reported similar
catalysts which produced crystalline versions of
polymers such as polystyrene, polymethylmethacrylate and polypropylene of high molecular weight.
199
These new materials were effectively the last links
in establishing the molecular structure criteria for
crystallizable polymers.
The new forms of polyethylene contained linear
chains or chains with very few branch points. Natta
showed from crystallographic data that his
polymers, which had pendant side groups, had
structural regularity, i.e. stereoregularity.
The so-called isotactic polymer chain of the
R
I
polymer -CH-CH 2 - (e.g., polystyrene or polypropylene) has all the pendant groups on the same
side of the plane of the carbon atoms as shown in
Figure l(a). In the syndiotactic form, the pendant
groups alternate strictly on either side of the chain
as in Figure l(b). The atactic form has the pendant
groups placed at random on either side of the chain
as in Figure l(c). The Ziegler/Natta catalysts
produce long runs of either iso- or syndiotactic
chain sequences with much shorter sections of
atactic sequences. The isotactic chains are regular
and can fit into a crystal lattice; so can syndiotactic
chains, although the resulting structure will be
different. Polymers composed of long atactic
sequences do not crystallize because they do not
possess sufficient steric regularity to produce stable
nuclei from which crystals can grow or allow the
chain to fit into a crystal lattice. These forms of
polymer exist only as glasses in the solid state.
Thus, the principles governing the formation of
R
R
R
R
R
|al Isoiuclic
H
VH
H
VH
H
V
V (f
V H
V H
H
(hi SMHIIOUI
|c| ALICUC
V R
Figure 1. Schematic representation of isotactic, syndiotactic and atactic vinyl polymers.
Geoffrey Allen
200
crystals in polymeric substances include the
following:
(i) The chemical repeat unit plays a role similar
to discrete molecules in crystals of a normal
organic compound.
(ii) The molecular chains must have long
sequences of stereoregularity.
(iii) Linear polymer molecules facilitate crystallization.
(iv) The unit cell contains only a small number
of repeat units.
(v) More than one chain may pass through a
unit cell.
On this basis, crystallization in all known synthetic
or natural polymers can be rationalized in terms
of the structure of the molecular chains. Apart
from the fact that a polymer molecule passes
through a large number of consecutive unit cells,
the crystal lattice is analogous to that of a simple
organic compound.
Crystallization and melting
Conceptual difficulties with the phenomenon of
crystallization in polymeric materials arise from the
fact that with the exception of polymethylene oxide
-(CH 2 -O) n - and certain proteins, macroscopic
single crystals are very rarely encountered.
Polymers do not crystallize completely; the
submicroscopic crystallites are embedded in the
remaining amorphous matrix.
X-ray diffraction again proved useful in establishing these facts. Pioneering studies in the early 1930s
showed that when a polymer is cooled slowly from
the melt (i.e., the rubbery state) sharp diffraction
rings are observed, showing that the sample
contains randomly orientated crystals. When the
sample is held in the temperature range where the
sharp rings increase in intensity, crystallization
eventually ceases, as judged from the X-ray
intensities, and there is a residual amorphous halo
demonstrating that amorphous material remains.
If cooling is continued to very low temperatures
there is no change in the pattern, despite the fact
that the sample will have changed from a rough
leathery texture to a hard solid. On warming, the
crystalline diffraction pattern disappears over a
range of temperature, but there is a well-defined
temperature at which the last traces disappear. This
melting temperature limit is some 20°C above the
temperature of the maximum rate of crystallization.
Rapid cooling often results in no crystallization
despite transformation of rubber to a hard brittle
glassy material.
Since there must be volume changes accompanying crystallization of the sample, dilatometric
studies were made to parallel the X-ray observations. The pioneering results obtained by Bekkedahl12 in 1934 on natural rubber showed a
volume-temperature curve obtained at slow rates
of cooling with crystallization and a contraction in
volume occurring near 0°C. Warming from liquid
air temperature shows a second-order change in
the volume—temperature relation at —72°C,
followed by a first-order melting process in the
range 6 to 16°C which is accompanied by an
expansion of 2.7%. This agrees with the X-ray
determination of the melting temperature of the
crystallites. By contrast, when the sample is cooled
rapidly to liquid air temperature it remains
amorphous. On warming, only the second-order
transition at — 72°C is observed as the sample
changes from glass to rubber. Thus, the melting
point of natural rubber is ~ 16°C and its
glass-transition temperature — 72°C. A combination
of X-ray and volumetric data shows that
unstretched natural rubber, taken to its limit of
crystallization, still contains some 70% of amorphous material.
Further work by Wood and Bekkedahl13
revealed behaviour which is known now to be
typical of the crystallization of many polymer
systems. First, the rate of crystallization showed a
maximum at about 0°C and the rate decreased on
further cooling—behaviour which is characteristic
of a system controlled by nucleation. Second, the
temperature range of melting was found to be a
function of the temperature at which crystallization
occurred. As the temperature of crystallization is
lowered, the temperature of melting of the crystals
produced also falls but the temperature range over
which melting is observed becomes greater, i.e.,
the melting is more diffuse. Under all conditions,
melting only begins some 5-10°C above the
crystallization temperature. To obtain the highest
melting point, crystallization must be carried out
above the temperature at which the optimum rate
of crystallization occurs. Since this is a region
where crystallization is slow, this effect has led to
Crystallinity in polymers
various melting points being reported for the same
material.
Taken together, these observations of melting
point ranges and incomplete crystallization mean
that when crystallization ceases, an equilibrium
state is not reached between the amorphous phase
and the crystalline phase. There will be a
distribution of crystallites of different degrees of
imperfection and also of different sizes. Because of
surface energy considerations, the smaller crystals
and more imperfect crystals will have lower melting
points. Further, there may be entanglements and
other constraints (for example, high viscosity) in
the amorphous phase which prevents reorganization
and recrystallization to produce an array of more
stable crystallites.
Polyethylene has provided further insights into
the nature of crystallization in polymers. Since
normal paraffins, CnH2n + 2, are well-defined chemicals, certainly up to C 94 H 190 , and within the series
their melting behaviour is very similar to that of
normal organic substances, it is possible to estimate
by extrapolation the melting point of an infinitely
long chain.
n
Tm°C
30
44
62.1
86.2
94
114.2
CO
140
The result is that polyethylene of high molecular
weight should melt at about 140°C. This result was
known at a time when polyethylene was manufactured from ethylene by a high-pressure process
using a free radical catalyst. Unfortunately, the
melting point of the product was only 115°C, well
below the estimate for a very long linear chain.
Furthermore, the polyethylene showed a limited
degree of crystallizability. From X-ray and
spectroscopic studies it was concluded that
polyethylene molecules made by this process are
highly branched. The whole issue was resolved
when Ziegler's new catalyst, which synthesized
chains with a very much lower degree of branching,
yielded highly crystalline products, initially with
melting temperatures of about 135°C and later in
the region of 140°C.
Now a strict comparison could be made of the
crystallization and melting behaviour of a polymer
201
with its discrete short-chain homologues, in this
case the n-paraffins. Figure 2(a) shows volumetemperature curves for C 44 H 90 and C 94 H 190 , and
Figure 2(b), curves for an unfractionated linear
polyethylene sample of number-average molecular
weight 32000. The dilatometric results give
evidence that it is possible to obtain melting curves
in polymers that are comparable with those of
single substances of low molecular weight. The
temperature at which the last traces of crystallinity
disappear is clearly defined for each sample. The
fact that the polymer sample contains a distribution
of chain lengths as well as some branched molecules
and a distribution of crystal imperfections does
broaden the melting range. Comparison of the
results for the polymer sample which was cooled
slowly from the melt, with the same sample
carefully crystallized at 130°C for 40 days, shows
that the latter sample attained a higher degree of
crystallinity. Both samples gave Tm = 140°C.
Thus, the differences in crystallization and
melting between substances of low molecular
weight and polymers is one of degree rather than
kind.14 Fusion in polymers is also a first-order
thermodynamic process. However, the main
difference is that polymers crystallize only in part.
This results in the observation of a glass transition,
with second-order character, in the residual rubber
phase at a temperature much lower than Tm.
Fibre formation is one of the most important
features of crystallizable polymers. The early X-ray
workers on cellulose noted that the crystallites
were orientated with their long axis parallel to the
fibre axis. In fact, the drawing of the fibre to align
the polymer molecules along the fibre axis and
hence promote the growth of orientated crystallites
is an essential feature of all synthetic fibre
processes, nylon, terylene, polypropylene, rayon,
etc. There are many natural examples, including
cotton, wool and silk. However, although these
systems attain quite high degrees of crystallinity
and because of crystallite orientation the bulk
properties are anisotropic, exactly the same
concepts of crystallization apply.
Crystallization and morphology
Crystalline polymers have the capacity to present a
complex hierarchy of morphologies extending from
Geoffrey Allen
202
T(°C)
106 108 110 112
^< ii6 118
11
<^-
14
•• 18
C
12
C
H
44 90
94 H 190
J
L
16
1
o
<u
10
CO
8•
J
6
(Q)
85
•o
o
•
14
•
o
12
86 87 88 89 90
i
I
i
i
1.3
1.25
o
o
d>
E
1.2
1.15
o
Specil
o
1.1
1.05
(b) 0.95
-40-20 0 20 40 60 80 100 120 140160 180
T (°C)
Figure 2. (a) Fusion of n-hydrocarbons. Plot of dilatometric scale reading against temperature for C 44 H 90 and
C 94 H 190 . (b) Specific volume-temperature relations for an unfractionated linear polyethylene sample. Slowly cooled
from melt o; isothermally crystallized at 130°C for 40 days, then cooled to room temperature •.
Crystallinity in polymers
203
unit cell dimensions, to crystallites in fibres and
isotropic substances of the order of 100-1000 A,
on to polycrystalline aggregates extending up to a
millimetre or so. In rare instances, macroscopic
single crystals can be obtained. At the molecular
level an individual chain may pass once or even
twice through a unit cell. The observation of
limited degrees of crystallinity attained in bulkcrystallized materials and estimates of crystallite
size suggests that a long molecule may emerge from
a crystallite, have a coiled segment embedded in
an amorphous domain and then return to the same
crystallite or enter a different one.
Thus, for many years, the accepted morphological structure of an unorientated, crystallized
polymer was the 'fringed micelle' model. 15 The
long molecules meander through a two-phase
structure in which the randomly arranged crystallites are bundles of orientated segments of polymer
chain some 100-1000 A long and the amorphous
domains are tangles of coiled chain. The model,
largely due to Bunn, is shown in Figure 3. It
incorporates the possibility that one molecule can
traverse several crystallites and the intervening
amorphous domains. Fibre formation is envisaged as
a straightening out of the chain and a consequent
growth of crystals orientated in the direction of
draw.
In 1945, Bunn 16 reported a larger scale of
ordering, following a study of polyethylene in a
polarizing optical microscope. These entities,
called spherulites, are micrometers in size, consisting
of approximately spherical arrays of equivalent
radiating crystalline units. It was not obvious how
(b)
Figure 4- Polyethylene lamellae crystallized from solution: (a) lozenge-shaped; (b) truncated lozenge-shaped.
(Courtesy of Dr. D. C. Bassett.)
Figure 3. Schematic representation of the fringedmicelle model (after Bunn).
the small crystallites of the fringed micelle model
could give rise to these structures since the
spherulites were orders of magnitude larger. Even
more curious and more difficult to accommodate
in the model was the observation deduced from
the optical birefringence that the polyethylene
chain axis was tangential rather than radial, i.e.,
perpendicular to the direction of the radiating
fibrils.
204
Geoffrey Allen
This difficulty remained until 1957 when
studies were made of the new linear (i.e.,
unbranched) polyethylene crystallized as individual
crystals from dilute solution. Examination in a
transmission electron microscope showed the
crystals to be lozenge-shaped, about 100 A in
thickness and several micrometres in length.
Keller17 demonstrated that within the crystal the
molecular chain axis was roughly perpendicular to
the plane of the lamella. Since the molecules were,
on average, about 1 urn long, Keller deduced that
they could be accommodated in a 100 A thick
crystal only by the chain folding back on itself
repeatedly at the crystal surfaces. These results
triggered a major international research effort on
individual lamellae and aggregates of lamellae
grown from linear polymers in solution.
Much work has been done on individual
polyethylene lamellae to validate the remarkable
finding by Keller. Electron diffraction has confirmed
that the c-axis of the unit cell is close to
perpendicular to the plane of the crystal and thus
so is the chain axis. It is accepted that each polymer
molecule folds back upon itself many times at the
upper and lower crystal surfaces (the 'fold
surfaces') rather like a Chinese cracker. However,
there is still controversy over the exact nature of
the fold. The Keller school argues that most of the
folds are tight, having four CH2 groups per turn
and molecular models show that this is quite
possible. The Flory school, mainly devoted to
thermodynamic studies, favours a model in which
a molecular chain at a surface may leave the crystal
and not necessarily return to an adjacent site via a
tight fold—a switchboard model18 in which some
folds contain a large number of carbon atoms to
re-enter the crystal at a more distant site.
Nevertheless in both models the polymer crystal
can be considered to have two components with
different properties—a well-ordered crystalline
interior plus lamellar surfaces which incorporate
the fold planes. We know now that many polymer
chains adopt folded-chain conformations in
lamellar structures. Keller himself19 has recently
recorded the history of his discovery and the
stormy debates which followed.
The form of single-crystal lamellae obtained
from a very dilute solution of polyethylene in
xylene at lower temperatures is the simple lozenge
shape with {110} growth faces. At higher
temperatures these crystals develop {100} faces
which give the lamellae the appearance of
truncated lozenges. Both forms are shown in Figure
4. Because the monolayer crystal has the c-axis of
the unit cell perpendicular to the plane of the
crystal and the crystal is bounded by growth faces
of low crystallographic index, the crystal habit is
orthorhombic, reflecting the symmetry of the unit
cell. In the same way, hexagonal monolayer crystals
are produced by polyoxymethylene and isotactic
polystyrene because they have hexagonal unit cells.
Detailed studies show that all these ' single crystals'
are actually multiple twins because the crystal habit
referred to above exists in the lamellae in a number
of crystallographic regions, each associated with a
particular growth face.
Single lamellae can only be obtained under
carefully regulated conditions. More generally,
crystallization from solution and sometimes from
the melt produces multilayered lamellar aggregates.
These multilayered aggregates result from screw
dislocation imperfections in the crystal growth
caused by chain folds which are staggered and
which give rise to the proliferation of layers. Where
such defects are limited by crystallization conditions
and slow growth occurs in solutions of moderate
concentration or where growth occurs in melts
which are only slightly supercooled, well-ordered
multilayered crystals can be obtained.
More usually in polymers, screw dislocation
defects and the splaying of lamellae generate
sheaf-like structures and ultimately the spherically
symmetrical spherulites which are the building
blocks of the morphology of polymers crystallized
in bulk. Spherulites and the arrangement of
lamellae in spherulites continue to be studied
extensively to elucidate the relation between their
structure and the macroscopic properties of the
material.
Before proceeding with crystallization in the
polymer melt we must note that spherulites are not
found exclusively in polymeric substances; they are
observed in many organic and inorganic materials
where crystallization occurs from a viscous melt.
However, the formation of spherulites is still not
well understood. In polymer systems such
understanding is made more difficult by the fact
that the direct transmission electron microscopic
observations made on individual lamellae grown
from solution cannot be applied directly to
lamellae grown from the melt. Specimens of
melt-crystallized materials have to be prepared by
Crystallinity in polymers
205
Direction of growth
Figure 6. A model lamellar fibril in a polyethylene
spherulite (after Takayanagi). The a, b, c axes depict the
orientation of the unit cell along the spiralling fibril.
Figure 5. Sheaf-like aggregates crystallized from the melt
in a blend of linear and branched polyethylene at 125°C.
(Courtesy Dr. D. C. Bassett.)
crystallizing thin films or by sectioning bulkcrystallized samples. It is not certain that the forms
produced by crystallization in a very thin film are
representative of those produced in bulk. Neither
is it certain that sectioning does not deform the
structures. Lamellar structures in bulk often have
to be deduced with the aid of two less direct
methods. Staining of samples using chlorosulphonic
acid or osmium tetroxide is one technique which
was developed originally for biological specimens.
The second technique involves a potassium
permanganate/sulphuric acid etching reagent which
selectively removes disordered material from the
surface of a specimen so that the ordered structural
features are revealed.
Bassett and his colleagues20 have provided an
insight into the origins of spherulites by crystallizing
polymers under conditions where the nucleation
density is very high and the volume available for
growth constrained so that mature spherulites do
not develop. At high crystallization temperatures,
sheaf-like structures can be seen. Figure 5 shows
the lamellar structure of a sheaf-like 'axialite'
grown from a polyethylene melt. When these
structures grow in an unconfined sample it is quite
possible that the lamellae will branch through the
mechanism of screw dislocation and then splay
apart.
Unfortunately it is difficult to quench polyethylene samples and freeze in structures developed
through these stages of growth. However, in
isotactic polystyrene it is observed that lamellae
branch through screw dislocations and this
generates spiral structures containing several
layers. Successive layers then splay apart. Since
screw dislocations develop on all growth faces this
results in three-dimensional growth. If these
mechanisms hold for the growth of the sheaf-like
polyethylene structures, eventually all solid angles
will be filled and a spherulite form produced. It is
widely believed that axialite sheaves differ in degree
only from spherulites.
Spherulite structures observed in several different
polymers have common features. By examining the
edge of a spherulite it can be shown that the thicker
lamellae develop first and, in doing so, enclose
columns of melt which later crystallize isothermally
near the centre of the spherulite or when the sample
is quenched, near the tips of the growing lamellae.
The forms of the overall structures observed are
dictated by the early-formed, thicker or so-called
dominant lamellae. The lamellae that form later are
thinner and in-fill the already established dominant
spherulite frame at interstitial sites. They are
termed subsidiary lamellae.
Using solvent extraction, it has been shown that
in polyethylene samples the longest, most linear
molecules crystallize first. Thus, they tend to be
located in the dominant lamellae. Shorter molecules
crystallize isothermally within the frame of
dominant lamellae and shorter chains still, in
regions which do not crystallize except on
quenching. Chains which do not crystallize form
a tenuous amorphous matrix. This molecular
fractionation influences macroscopic properties.
The regions in which material of lower molecular
weight is concentrated provide relatively easy
206
Geoffrey Allen
routes through which cracks propagate. This is one
reason why spherulite size and structure are
considered to be important in the control of bulk
properties of crystalline polymers.
In all structures, the observation made over 40
years ago by Bunn, that the polymer chain axes lie
tangential and not radial to the spherulite structure,
is explained by Keller's folded chain. The chains
are folded within the spiralling lamellae with the
chain axis perpendicular to the growing faces, as
shown schematically in Figure 6.
14. L. Mandelkern (1989) In Comprehensive Polymer
Science, edited by G. Allen and J. H. Bevington.
(Pergamon Press) 2,, 363.
15. C.W. Bunn (1953) Fibres from Synthetic Polymers,
edited by R. Hill. (Amsterdam: Elsevier), p. 240.
16. C.W. Bunn and T.C. Alcock (1945) Trans Faraday
Soc 41, 317.
17. A. Keller (1957) Philos Mag [8] 2, 1171; (1958)
Discuss Faraday Soc 25, 114.
18. P. J. Flory (1989) In Comprehensive Pofymer Science,
edited by G. Allen and J. H. Bevington (Oxford:
Pergamon Press).
REFERENCES
1. J. Gough (1805) Mem Lit Phil Soc Manchester 2, 288.
2. M. Polanyi (1921) Naturwiss 9, 228.
3. M. Polanyi (1962) In 50 Years of X-ray Diffraction
edited by P.P. Ewald. Vosthoek.
4. J.R. Katz (1925) Kolloid Z. 37, 19.
19. A. Keller (1991) In Sir Charles Frank: An Eightieth
Birthday Tribute, edited by R.G. Chambers, J.E. Enderby,
A. Keller, A.R. Lang and J.W. Steels (Bristol: Adam
Hilger), p. 265.
20. A.S. Vaughan and D.C. Bassett (1989) In Comprehensive Polymer Science, edited by G. Allen and J.H.
Bevington. (Oxford: Pergamon Press), 2, 415.
5. H. Staudinger and M. Luthy (1925) Helv Chim Acta
8,65.
6. K.H. Meyer and H. Mark (1928) Berichte B61, 593,
1932, 136, 1939.
7. W.T. Astbury (1938) Tram Faraday Soc 34, 378.
8. L. Pauling, R.B. Carey and H.R. Branson (1951) Proc
Nat Acad Sci USA 37, 205.
9. F.H.C. Crick and J.D. Watson (1953) Nature 171,
737, 964.
10. K. Ziegler, E. Holzkamp, H. Brell and H. Martin
(1955) Angew Chem 67, 541.
11. G. Natta, I.W. Bassi and P. Corradini (1955)
Makromol Chem 18-19, 455.
12. N. Bekkedahl (1934) J Res Nat Bur Stand 13, 411.
13. L.A. Wood and N. Bekkedahl (1946) ] Appl Phys
17, 362.
Author's biography:
Geoffrey Allen is a Fellow of the Royal Society
and a Foreign Member of the Engineering Academy
of Japan. He was formerly Professor of Chemical
Technology at the Imperial College of Science and
Technology. He then became Chairman of the
Science and Engineering Research Council and
following that appointment was Research and
Engineering Director of Unilever for nine years.
He is now Executive Adviser to Kobe Steel Ltd.
He has written numerous papers on the thermodynamic properties of polymers and on the
application of structural techniques to plastics and
rubbers.