Ideal Gas Law: Chemistry Presentation
advertisement

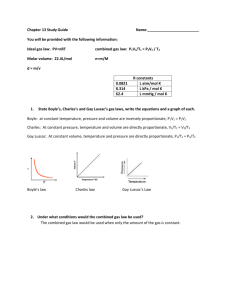
Lesson 4: Ideal Gas Law This lesson combines all the properties of gases into a single equation. Ideal Gas Law Combining Boyle’s and Charles’ laws allows for developing a single equation: P*V = n*R*T P = pressure V = volume n = number of moles R = universal gas constant (we’ll get to that in a minute…) T = temperature Ideal Gas Law P*V = n*R*T This is one of the few equations in chemistry that you should commit to memory! By remembering this single equation, you can predict how any two variables will behave when the others are held constant in an IDEAL GAS Gas Constant The Ideal Gas Law as presented includes use of the Universal Gas Constant. The value of the constant depends on the units used to define the other variables. Usually: 0.0821 L*atm/mol*K Practice How many moles of a gas at 100⁰C does it take to fill a 1.00 L flask to a pressure of 1.50 atm? PV=nRT (1.50 atm)(1.00 L) = n(0.0821 atm*L/mol*K)(373 K) n = 0.0490 mol Ideal Gas Law: Summary P*V = n*R*T Learn it! Use it! This single equation can be used to predict how any two variables will behave when the others are held constant. Another Example – Using mass Calculate the volume (in L) occupied by 7.40 g of NH3 at STP. Moles = 7.40 g /17 g (MM) = 0.44 mol Plug into PV = nRT Or the easier way!!! You know at STP, a gas takes up 22.4 L So 0.44 mol x (22.4 L / 1 mol) = 9.74 L PV = nRT ….x 2! A small bubble rises from the bottom of a lake, where the temperature and pressure are 8°C and 6.4 atm, to the water’s surface where the temperature is 25°C and the pressure is 1.0 atm. Calculate the final volume (in mL) of the bubble if its initial volume was 2.1 mL. We have two pressures, two temperatures, and one volume (with the other one we need to find) P1V1 n1T1 P2V2 n2T2 R is left out since it’s the same on both sides and will cancel itself out! Technically, Boyle’s and Charles’ Laws are this equation PV = nRT…x 2!!! Now, we just plug everything in (assume n stays constant since it’s not mentioned) P1 = 6.4 atm, V1 = 2.1 mL, T1 = 281 K P2 = 1.0 atm, V2 = ?, T2 = 298 K (6.4*2.1)/281 = (1.0x)/298 x = 14 mL Double PV=nRT Example #2 A gas initially at 4.0 L, 1.2 atm, and 66°C undergoes a change so its final volume and temperature are 1.7 L and 42°C, respectively. What is the final pressure assuming the number of moles remains unchanged? Density Calculations We can rearrange the ideal gas equation to find density or molar mass: PV = nRT, and n = mass (m)/MM (M) Rearrange PV = nRT… Substitute in m/M (n/V) = P/RT m/MV = P/RT Since density is mass/volume…. D = PM/RT Example Calculate the density of CO2 in g/L at 0.990 atm and 55°C. d = PM/RT d = (0.990 atm)(44.01 g/mol) / (0.0821 L*atm/mol*K)(328 K) d = 1.62 g/L Non-Ideal Gases Ideal gas law does NOT describe gases in everyday behavior PV=nRT also called the kinetic molecular theory We use it as an approximation When do gases not obey the ideal gas law: 1. High pressure 2. Very Low temperatures Ideal Gases don’t exist Molecules do take up space All matter has volume There are attractive forces otherwise there would be no liquids Real Gases behave like Ideal Gases When the molecules are far apart They take a smaller percentage of the space Ignoring their volume is reasonable This is at low pressure Real Gases behave like Ideal gases when When molecules are moving fast. Molecules are not next to each other very long Attractive forces can’t play a role. At high temp. Far above boiling point. Effect of Pressure Molecule size because they are close together Intermolecular forces stick molecules together 17 Effect of Temperature 18 Van der Waal’s equation 2 n P obs + a x V - nb nR T V a is a number that depends on how much the molecules stick to each other – constant that corrects for pressure b is a number that determined by how big the molecules are – constant that corrects for volume 19 Real Gas Example (van Der Waals) Given that 3.50 mol of NH3 occupy 5.20 L at 47°C, calculate the pressure of the gas using (a) the ideal gas equation and (b) the van der Waals equation) (a) PV = nRT P(5.20 L) = (3.50)(0.0821)(320 K) P = 17.7 atm Real Gas Example (van Der Waals) Given that 3.50 mol of NH3 occupy 5.20 L at 47°C, calculate the pressure of the gas using (a) the ideal gas equation and (b) the van der Waals equation) (b) [P+ (an2/V2)](V-nb) = nRT a = 4.17 atm*L2/mol2 b = 0.0371 L/mol [P +(4.17*(3.502))/(5.202) = (3.50)(0.0821)(320) P = 16.2 atm