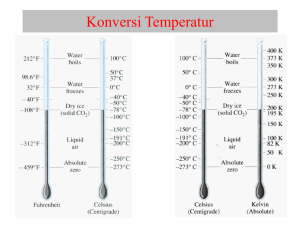
thermal_power_conversionMOD
advertisement

INTRODUCTION TO RENEWABLE ENERGY course code IE3320 subject: THERMAL POWER CONVERSION N. Woudstra e-mail: n.woudstra@wbmt.tudelft.nl Course material: • Renewable energy; second edition, Godfrey Boyle, Open University; ISBN 0-19-926178-4 chapter 2 section 2.9 and 2.10 (solar thermal energy) chapter 9 section 9.1, 9.2 and 9.3 (geothermal energy) • Copies of slides (blackboard) Additional material: • Fundamentals of Engineering Thermodynamics; third edition, Moran & Shapiro, ISBN 0-471-97960-0 THERMODYNAMIC LAWS thermodynamic laws are based on experience FIRST LAW: ‘energy can neither be lost nor originated from the nothingness’ [basis for energy balances] SECOND LAW: ‘work can be transformed completely into heat, but heat cannot be transformed completely into work’ [basis for definition of the thermodynamic temperature scale (Kelvin scale), entropy, exergy, ……etc.] SECOND LAW OF THERMODYNAMICS “work can be converted completely into heat, but heat cannot be converted completely into work” hot reservoir at temperature TH efficiency of thermal power cycle: th QH thermal power cycle QC cold reservoir at temperature TC W W QH QC QH QH efficiency of a reversible cycle depends only on temperatures of heat transfer to and from the system the thermodynamic temperature scale (Kelvin scale) has been defined such that: QH QC TH TC T 1 C Q1 TH TH T then: work from a reversible power cycle becomes: Wrev 1 C QH TH TC the term 1 is generally known as the Carnot efficiency TH th, rev. SECOND LAW OF THERMODYNAMICS efficiency of a reversible cycle: th, rev. 1 hot reservoir at temperature TH because of losses within the cycle the efficiency of an irreversible cycle will always be lower, QH thermal power cycle QC cold reservoir at temperature TC TC TH W therefore: or: with: th, irrev. th, irrev. 1 TC TH TC ex, intern. 1 TH ex, intern. then: work from an irreversible power cycle becomes: Wirrev Wrev T Wirrev ex, intern. 1 C QH TH in general temperatures of heat transfer to and from power cycles are not constant this complicates the application of this equation TEMPERATURE OF HEAT TRANSFER heat transfer to fluid without phase change (liquid or gas) T temperature increase due to heat transfer T Q heat transfer to fluid with phase change (from liquid to gas) evaporation (phase change) Q temperature increase in liquid phase and gas phase constant temperature during evaporation (phase change) WORK FROM HEAT OF A FLUID FLOW as: Wrev 1 TC QH TH then the reversible work from an infinitesimal amount of heat becomes: TC dWrev 1 dQH TH T and from a fluid flow with varying temperatures: Wrev 1 C dQH TH suppose: temperature of cold reservoir is ambient temperature T0 T0 1 dQH TH in out then: Wrev the thermodynamic equivalent temperature of heat transfer to the fluid TH is by definition: Wrev out T0 T 1 QH 1 0 dQH TH TH in WORK FROM HEAT OF A FLUID FLOW Wrev out T0 T 1 QH 1 0 dQH TH TH in it appears that the equivalent temperature of heat transfer TH can be determined with the following (simple) equation: TH H hout hin S sout sin in case of ideal gas with constant specific heat (without phase change) this equation can be transformed into: TH hout hin Tout Tin T sout sin ln out Tin this equation can also be used to determine TC when transfer from the system occurs at varying temperatures: TC hout hin Tout Tin T sout sin ln out Tin POWER FROM THERMAL POWER CYCLE work from a thermal power cycle can be determined by applying the following equation: TC Wirrev ex, intern. (1 TH ) QH as power P is work per unit of time, and Q a heat flow, then the following equation results for the power from a thermal power cycle: Pcycle ex, intern. (1 TC ) QH TH when heat is generated in a boiler, the heat flow to cycle becomes: QH th, boiler m, F LHVF with: th, boiler LHVF thermal efficiency boiler (-) lower heating value of fuel (kJ/kg) efficiencies (internal efficiency as well as boiler efficiency) strongly depend on type and scale of the considered system: th, boiler 0.90 à 0.95 for large steam boilers: for large steam turbine cycles: ex, intern. 0.80 EXERGY OF HEAT definition: exergy is the maximum theoretical work that can be derived from an amount of energy (in a well defined system), using the environment as reservoir for heat (and matter) exergy of heat at temperature T: T ExQ Wrev 1 0 Q T (according to the definition the temperature of the cold reservoir is T0 ) the exergy of heat from a fluid flow = reversible work from the heat that can be extracted from the fluid flow: T ExQH Wrev 1 0 QH TH THERMAL POWER CYCLE simple steam cycle 1 1 = boiler 2 = turbine 2 3 = condenser 1 4 = condensate pump 7 2 5 = deaerator 8 6 6 = feedwater pump 7 5 3 4 9 10 8 3 6 5 4 7 = heat sink 8 = cooling water pump THERMAL POWER CYCLE standard steam cycle standard steam cycle • superheated steam • without steam reheat • without feedwater preheat T life steam conditions: p3 40 bar ; T3 530 C h h 3513.1 129.3 TH 3 1 s3 s1 7.1774 0.4353 502 K (229 C) TC Tcond 30 C (303 K) 3 TH Tcond. 1 5 Wirrev T ex, intern. (1 C ) QH TH with: ex, intern. 0.75 303 th 0.75 (1 ) 0.297 502 th 2 4 x minimum s THERMAL POWER CYCLE low temperature saturated steam cycle low temperature steam cycle • saturated steam • without steam reheat • without feedwater preheat life steam conditions: p3 3.0 bar ; T3 134 C h h 2724.7 129.3 TH 3 1 s3 s1 6.9909 0.4353 396 K (123 C) TC Tcond 30 C (303 K) T Wirrev T ex, intern. (1 C ) QH TH with: ex, intern. 0.75 303 th 0.75 (1 ) 0.176 396 th 2 TH Tcond. 3 1 5 4 x minimum s THERMAL POWER CONVERSION AND RENEWABLE ENERGY HEAT SOURCES: (biomass combustion gasification + combustion) geothermal heat low temperature heat solar heat (low temperature heat (flat plate collectors)) high temperature heat parabolic concentrators solar central receiver (solar tower) SOLAR HEAT SOLAR HEAT Concentrated solar radiation enables high temperature of heated liquids SOLAR HEAT SOLAR ELECTRIC GENERATING SYSTEM (SEGS) SOLAR HEAT GEOTHERMAL HEAT GEOTHERMAL HEAT Natural steam from the production wells power the turbine generator. Steam is condensed by evaporation in the cooling tower and pumped down an injection well to sustain production. DRY STEAM POWER PLANTS Steam plants use hydrothermal fluids that are primarily steam. The steam goes directly to a turbine, which drives a generator that produces electricity. This is the oldest type of geothermal power plant. It was first used at Lardarello in Italy in 1904, and is still very effective (>700 MWe). Steam technology is used today at The Geysers in northern California, the world's largest single source of geothermal power. FLASH STEAM POWER PLANTS Hydrothermal fluids above 360ºF (182ºC) can be used in flash plants to make electricity. Fluid is sprayed into a tank held at a much lower pressure than the fluid, causing some of the fluid to rapidly vaporise, or "flash." The vapour then drives a turbine, which drives a generator. BINARY-CYCLE POWER PLANTS Most geothermal areas contain moderate-temperature water (below 200 ºC). Energy is extracted from these fluids in binary-cycle power plants. Hot geothermal fluid and a secondary (hence, "binary") fluid with a much lower boiling point than water pass through a heat exchanger. Heat from the geothermal fluid causes the secondary fluid to flash to vapour, which then drives the turbines. Moderate-temperature water is by far the more common geothermal resource, and most geothermal power plants in the future will be binary-cycle plants.