Clouds
advertisement
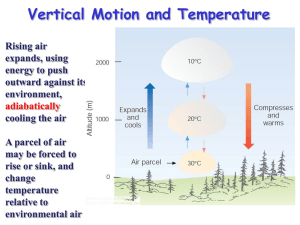
METR125: Cloud Microphysics – Nucleation of water vapor condensation Wallace and Hobbs, Sections 6.1 and 6.4 Menglin Jin Prof. Jin and Prof. Wallace, AMS Courtesy: Steve Platnick, NASA Warm Cloud Processes Cold Cloud Processes Rain Drops, Cloud Droplets, and CCN • relative sizes of rain drops, cloud drops, and CCN: – raindrops - 2000 μm = 2 mm • fall at a speed of 4-5 ms-1 – cloud drops - 20 μm = 0.02 mm • remain suspended in the air – CCN - 0.2 μm = 0.0002 mm • remain suspended in the air • To get a droplet (20 μm) to grow to raindrop size (2000μm) it must increase in size by a factor of 100 (two orders of magnitude): – 2000μm/20μm = 100 • this occurs in about 30 minutes in a thunderstorm!!! • this is like a 150 lb person growing in size to 15,000 lbs in half an hour!!! • Q: How does this happen?? Video: cloud formation in Tucson • http://www.youtube.com/watch?v=NiCSk1 zxMEs Timelapse of Tucson cloud formations Q: How and why do clouds form on some regions and not on others???? Q: Why does the atmosphere sometimes produce stratus clouds (thin layered) while other times we get cumulus, or cumulonimbus clouds to form?? The answer is largely related to the concept of atmospheric stability..... Cloud Development - stable environment Consider this simple situation of a marble in the bottom of a bowl: if you push the marble up the side of the bowl, it will fall back down to the bottom, to it's original position Stable air (parcel) - vertical motion is inhibited if clouds form, they will be shallow, layered clouds like stratus Cloud Development - unstable environment If the marble is on the top of the bowl and you give it a little push, it rolls off the bowl.... does NOT come back to its original position This is an unstable situation Unstable air (parcel) - vertical motion occurs •commonly produces cumulus, cumulonimbus clouds So, the question becomes, how does one determine the stability of the atmosphere? Assessing Atmospheric Stability – To determine whether or not a parcel will rise or sink in the atmosphere, one must compare the parcels temperature (Tp) with that of the environment (Te) at some altitude: – if Tp > Te what will the parcel do? It will rise since it is less dense than the air – if Tp = Te what will the parcel do? It will remain at that location – if Tp < Te what will the parcel do? IT WILL SINK SINCE DENSER THAN THE AIR Assessing Atmospheric Stability to assess stability, what two pieces of information do we need ? We need to know •the vertical temperature profile and • temperature of the parcel of the air Determining Air Parcel Temperature: Rising air parcels and adiabatic cooling • consider a rising parcel of air -->> •As the parcel rises, it will adiabatically expand and cool • adiabatic - a process where the parcel temperature changes due to an expansion or compression, no heat is added or taken away from the parcel the parcel expands since the lower pressure outside allows the air molecules to push out on the parcel walls since it takes energy for the parcel molecules to "push out" on the parcel walls, they use up some of their internal energy in the process. therefore, the parcel also cools since temperature is proportional to molecular internal energy Determining Air Parcel Temperature: Rising air parcels and adiabatic cooling • consider a sinking parcel of air -->> As the parcel sinks, it will adiabatically compress and warm • the parcel compresses since it is moving into a region of higher pressure • due to the parcel compression, the air molecules gain internal energy • hence, the mean temperature of the parcel increases Dry adiabatic lapse rate • As a parcel of air rises, it cools, but at what rate??? •rate of temperature change with height is called the lapse rate •units of lapse rate are °C km-1 First case: an unsaturated parcel of air unsaturated parcels cool at a rate of 10°C km-1 - this is called the dry-adiabatic lapse rate What will be the parcel's temperature be at 1 km? Answer: 30 degrees Celsius Dry adiabatic lapse rate, cont • What will be the parcel's temperature be at 2 km? Answer: 20C Moist Adiabatic Lapse Rate For a saturated parcel of air, i.e., when its T=Td, then it cools at the moist adiabatic lapse rate = 6°C km-1 What will be the parcel's temperature be at 3 km? ANSWER: 14C Moist Adiabatic Lapse Rate • What will be the parcel's temperature be at 4 km? Answer: 8C Moist Adiabatic Lapse Rate Why does the parcel cool at a slower rate (6 °C km-1) when it is saturated than at 10°C km-1 when it is unsaturated?? Answer: Because latent heat is released into the air parcel as vapor condenses into water The latent heat counteract the adiabatic cooling. Dry versus Moist-Adiabatic Process • the moist adiabatic lapse rate is less than the dry adiabatic lapse rate because as vapor condenses into water (or water freezes into ice) for a saturated parcel, latent heat is released into the parcel, mitigating the adiabatic cooling --> Absolute Stability Absolute Stability • • consider the diagram to the right, notice that: Tup < Tsp < Te – where Tup is the temperature of an unsaturated parcel = 0°C – Tsp = temperature of a saturated parcel = 10°C – Te = environmental temperature = 20°C. • • • • generally, notice that Te is always larger than Tsp and Tup at any level Hence, an unsaturated or saturated parcel will always be cooler than the environment and will sink back down to the ground This is an example of absolute stability The condition for absolute stability is: – Gd>Gm>Ge • • • • Gd is the dry adiabatic lapse rate (10°C km-1) Gm is the moist adiabatic lapse rate (6°C km-1) Ge is the environmental lapse rate (variable - 0°C km-1 in this case) Q: How would you characterize the stability of an inversion layer? Stability of Inversion Layers How would you characterize the stability of an inversion layer? They are absolutely stable see diagram to the right -->> note that the absolute stability criteria: Ge<Gm<Gd Atmospheric Instability and Cloud Development What determines the base (bottom) of a cloud?? Q: What determines the height to which the cloud will grow?? let's use the previous example of a rising air parcel -->> Q: On this diagram, where is cloud base? Q: On this diagram, where is cloud top? LFC – level of free convection where in atmosphere The environment temperature decreases faster Than the moist adiabatic lapse rate LCL –Listed Convection Level Atmospheric Instability and Cloud Development • • • • • Q: On this diagram, where is cloud base? A: Where the parcel reaches saturation - 2 km Q: On this diagram, where is cloud top? A: Where the parcel will no longer be able to rise - 9 km Here, Tp = Te - this is often referred to as the equilibrium level (EL) Example Microphysical Measurements in Marine Sc Clouds (ASTEX field campaign, near Azores, 1992) Data from U. Washington C-131 aircraft PHYS 622 - Clouds, spring ‘04, lect.2, Platnick Cloud Droplet Formation S. Platnick notes Water Cloud Formation Water clouds form when RH slightly greater than 100% (e.g., 0.3% supersaturation). This is a result of a subset of the atmospheric aerosol serving as nucleation sites (to be discussed later). Common ways for exceed saturation: 1. Mixing of air masses (warm moist with cool air) 2. Cooling via parcel expansion (adiabatic) 3. Radiative cooling (e.g. ground fog, can lead to process 2) PHYS 622 - Clouds, spring ‘04, lect. 2, Platnick Concepts es(T) (T1,e1) e Radiative Cooling saturated Mixing (T2,e2) unsaturated T PHYS 622 - Clouds, spring ‘04, lect. 2, Platnick q, w, e, T of the mixed air • See textbook and notes • q= M1 M1+M2 W= e= T= q1 + M2 M1+M2 q2 Saturation Vapor Pressure (Clausius-Clapeyron equation) At equilibrium, evaporation and condensation have the same rate, and the air above the liquid is saturated with water vapor; the partial pressure of water vapor, or the Saturation Vapor Pressure (es) is: es (T) es Ttr e Air and water vapor T T Water L 1 1 ( ) R v T Ttr Where Ts=triple point temperature (273.16K), L is the latent heat of vaporization (2.5106 J/kg), es(Ttr) = 611Pa (or 6.11 mb). Rv is the gas constant for water vapor (461.5 J-kg1-K1). specific Platnick Saturation Vapor Pressure An approximation for the saturation vapor pressure (Rogers & Yau): e s (T ) Ae Over liquid water: L = latent heat of vaporization/condensation, A=2.53 x 108 kPa, B = 5.42 x 103 K. Over ice: L = latent heat of sublimation, A=3.41 x 109 kPa, B = 6.13 x 103 K. Platnick B T Rain Drops, Cloud Droplets, and CCN Processes for Cloud Droplet Growth • How does this happen?? • By: – condensation – collision/coalescence – ice-crystal process today Water Droplet Growth Condensation & Collision • Condensational growth: diffusion of vapor to droplet • Collisional growth: collision and coalescence (accretion, coagulation) between droplets PHYS 622 - Clouds, spring ‘04, lect.4, Platnick Water Droplet Growth - Condensation Flux of vapor to droplet (schematic shows “net flux” of vapor towards droplet, i.e., droplet grows) Need to consider: 1. Vapor flux due to gradient between saturation vapor pressure at droplet surface and environment (at ∞). 2. Effect of Latent heat effecting droplet saturation vapor pressure (equilibrium temperature accounting for heat flux away from droplet). PHYS 622 - Clouds, spring ‘04, lect.4, Platnick Cloud Droplet Growth by Condensation • Consider pure water in equilibrium with air above it C-C equation to calculate es Growth by Condensation Cloud Droplet Consider pure water in equilibrium with air above it: • then the RH = 100% • evaporation = condensation • vapor pressure (e) = saturation vapor pressure (es) • if evaporation > condensation, water is _________ • if evaporation < condensation, water is ________ • Now, a droplet surface is not flat, instead, it has curvature..... Q: how does curvature affect the evaporation/condensation process?? Flat versus Curved Water Surfaces Flat versus Curved Water Surfaces: curvature effect • • • • • • • • more energy is required to maintain the "curvature" of the drop therefore, the water molecules on the surface of the drop have more energy therefore, they evaporate more readily that from the flat water surface (compare the length of the red arrows) therefore: evaporation rate off curved surface > evaporation rate off of flat surface since air above both surfaces is saturated, then evaporation rate = condensation rate therefore, condensation rate onto droplet > condensation rate onto flat water surface therefore, esdrop > esflat therefore: – if RHflat = 100%, then RHdrop > 100% – the air surrounding the drop must be supersaturated!! • This is called the curvature effect Kelvin’s Equation Curvature Effect Curvature effect --> •notice that for the droplet to be in equilibrium (evaporation off drop = condensation onto drop), the environment must be supersaturated •also notice that the curvature effect is larger for smaller drops this makes sense since smaller drops have more curvature that larger drops Discuss: see text book Class activity-Curvature Effect • Q: what will happen to a drop 1.9 μm in size that is in a cloud where the RH is 100.05%? • Q: what will happen to a drop 1.9 μm in size that is in a cloud where the RH is 100.15%? Class activity-Curvature Effect • Q: what will happen to a drop 1.9 μm in size that is in a cloud where the RH is 100.05%? • Q: what will happen to a drop 1.9 μm in size that is in a cloud where the RH is 100.15%? QUESTIONS FOR THOUGHT: 1. At what relative humidity will pure water droplets of the following sizes grow by condensation: a. 10 microns b. 4 microns c. 1 micron 2. Explain why very small cloud droplets of pure water evaporate even when the relative humidity is 100%. Solution Droplets Note that the previous discussion is valid for a pure water drop • if a droplet is comprised of a solution - it can be in equilibrium with the environment at a much lower RH --> • this explains the formation of haze • This process of condensation will grow drops , but not to precipitation sizes (~ 2 mm) Q: So, if a droplet grows to some size by condensation, how can it continue to grow to precipitation size??? QUESTION FOR THOUGHT: • Haze particles can form when the relative humidity is less than 100%. Are these haze particles pure water droplets or solution droplets? Why? Köhler Curve How is amount of solution change water droplet formation? Give example. How different solution change water droplet formation? Give example. Extra reading about curvature and solutions effects!!! • http://www.shodor.org/os411/courses/411c/ module07/unit02/page03.html Droplet activated • Find it in text book P214 Water Droplet Growth - Condensation Growth slows down with increasing droplet size: large droplets : G s dr ~ env dt r Since large droplets grow slower, there is a narrowing of the size distribution with time. R&Y, p. 111 PHYS 622 - Clouds, spring ‘04, lect.4, Platnick rdry= 0.1 0.22 0.48 m Assumes supersaturation=0.05%, p=900 hPa, T=273K Water Droplet Growth - Condensation Diffusional growth summary: • Accounted for vapor and thermal fluxes to/away from droplet. • Growth slows down as droplets get larger, size distribution narrows. • Initial nucleated droplet size distribution depends on CCN spectrum & ds/dt seen by air parcel. • Inefficient mechanism for generating large precipitation sized cloud drops (requires hours). Condensation does not account for precipitation (collision/coalescence is the needed for “warm” clouds - to be discussed).