Mixing and Convection
advertisement

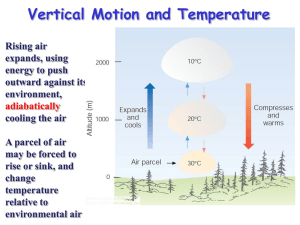
METR215 – Advanced Physical Meteorology Lecture 4: Mixing and Convection Texts: Rogers, R.R., and M. K. Yau, A Short Course in Cloud Physics, Pergamon Press, 1989. Modified from Steve Platnick Notes Cold Cloud Processes Warm Cloud Processes PHYS 622 - Clouds, spring ‘04, lect. 1, Platnick Water Cloud Formation Water clouds form when RH slightly greater than 100% (e.g., 0.3% supersaturation). This is a result of a subset of the atmospheric aerosol serving as nucleation sites (to be discussed later). Common ways for exceed saturation: 1. Mixing of air masses (warm moist with cool air) 2. Cooling via parcel expansion (adiabatic) 3. Radiative cooling (e.g. ground fog, can lead to process 2) Platnick Video: cloud formation in Tucson • http://www.youtube.com/watch?v=NiCSk1z xMEs Timelapse of Tucson cloud formations Q: How and why do clouds form on some days and not on others? Q: Why does the atmosphere sometimes produce stratus clouds (thin layered) while other times we get cumulus, or cumulonimbus clouds to form? The answer is largely related to the concept of atmospheric stability..... Assessing Atmospheric Stability to assess stability, what two pieces of information do we need ? We need to know • the vertical temperature profile •The temperature of parcel of the air Absolute Stability Absolute Stability • The condition for absolute stability is: – Gd>Gm>Ge • Gd is the dry adiabatic lapse rate (10°C km-1) • Gm is the moist adiabatic lapse rate (6°C km-1) • Ge is the environmental lapse rate (variable - 0°C km-1 in this case) Stability of Inversion Layers How would you characterize the stability of an inversion layer? They are absolutely stable see diagram to the right -->> note that the absolute stability criteria: Ge<Gm<Gd Formation of Subsidence Inversions • How does the stability change for a descending layer of air? Formation of Subsidence Inversions • How does the stability change for a descending layer of air? Absolute Instability • consider the diagram to the right, notice that at 2 km (or anywhere for that matter): Tsp > Tup > Te • Hence, an unsaturated or saturated parcel will always be warmer than the environment and will continue to ascend • This is an example of absolute instability • The condition for absolute instability is: – Ge>Gd>Gm • • • Ge is the environmental lapse rate (30°C km-1) Gd is the dry adiabatic lapse rate (10°C km-1) Gm is the moist adiabatic lapse rate (6°C km-1) Conditional Instability • consider the diagram to the right, notice that at 2 km (or anywhere for that matter): • Tup < Te < Tsp • The unsaturated parcel will be cooler than then environment and will sink back to the ground • The saturated parcel will be warmer than the environment and will continue to ascend • This is an example of conditional instability • The condition for conditional instability is: – Gd>Ge>Gm • Gd is the dry adiabatic lapse rate (10°C km-1) • Ge is the environmental lapse rate (7.8°C km-1) • Gm is the moist adiabatic lapse rate (6°C km-1) Conditional Instability - example • consider a parcel with a surface temperature and dew point of 30 °C and =14°C, respectively -->> • • the parcel is initially forced to rise in an environment where the environmental lapse rate (Ge) is 8°C km-1 up to 8 km. let's follow the parcel upward...... Conditional Instability - 1km the parcel is rising dry adiabitically (10°C km-1) as it is unsaturated note Tp < Te so something is forcing the parcel upward... onward to 2km .... Conditional Instability - 2km • the parcel has just become saturated • note Tp < Te so something is still forcing the parcel upward... • onward to 3km ... Conditional Instability - 3km the parcel is now rising moist adiabatically (6°C km-1) note Tp < Te so, something is still forcing it upward.... onward to 4km ..... Conditional Instability - 4km • the parcel is still rising moist adiabatically (6°C km-1) • note that now Tp = Te • what happens if the parcel is pushed upward just a little??? Conditional Instability - 5km and above Conditional Instability - 5km and above • note Tp > Te • A: it will rise on its own since now it is less dense than the surrounding environmental air • The height where Tp becomes equal to and then larger than Te is called the level of free convection • the parcel is still rising moist adiabatically (6°C km-1) • the parcel will continue to rise until: – Tp = Te • above that point, Tp < Te , so the parcel will rise no further • so, below 4 km where Tp < Te , the atmosphere is stable to parcel movement • above 4 km where Tp > Te , the atmosphere is unstable to parcel movement • this is an example of a conditionally unstable atmosphere... the condition is lifting the parcel above 4 km where it can then rise on its own Stability of the environment • To determine the environmental stability, one must calculate the lapse rate for a sounding • lapse rate = DT/DZ = T2-T1/Z2-Z1 • Since the environment is often composed of layers with different stabilities, it is useful to first identify these layers and then calculate their respective lapse rates • recall the stability criteria: • Ge < Gm - Absolutely stable • Gm < Ge < Gd - Conditional Instability • Gm < Gd < Ge - Absolutely unstable Stability of the environment • Characterize the stability of the layers in the sounding to the right --> • layer 1 • layer 2 • layer 3 • layer 4 • layer 5 • layer 6 Atmospheric Instability and Cloud Development What determines the base (bottom) of a cloud?? Q: What determines the height to which the cloud will grow?? let's use the previous example of a rising air parcel -->> Q: On this diagram, where is cloud base? Q: On this diagram, where is cloud top? Atmospheric Instability and Cloud Development • • • • • Q: On this diagram, where is cloud base? A: Where the parcel reaches saturation - 2 km Q: On this diagram, where is cloud top? A: Where the parcel will no longer be able to rise - 9 km Here, Tp = Te - this is often referred to as the equilibrium level Concepts of Mixing es(T) (T1,e1) e saturated Radiative Cooling Mixing (T2,e2) unsaturated T Hygrometric Chart - Isobaric mixing of two air samples Platnick q, w, e, T of the mixed air • See textbook and notes M2 • q= M1 q + M1+M2 W= e= T= 1 M1+M2 q2 Saturation Vapor Pressure (Clausius-Clapeyron equation) At equilibrium, evaporation and condensation have the same rate, and the air above the liquid is saturated with water vapor; the partial pressure of water vapor, or the Saturation Vapor Pressure (es) is: es (T) es Ttr e Air and water vapor T T Water L 1 1 ( ) R v T Ttr Where Ts=triple point temperature (273.16K), L is the latent heat of vaporization (2.5106 J/kg), es(Ttr) = 611Pa (or 6.11 mb). Rv is the gas constant for water vapor (461.5 J-kg1-K1). specific Platnick Saturation Vapor Pressure An approximation for the saturation vapor pressure (Rogers & Yau): e s (T ) Ae Over liquid water: L = latent heat of vaporization/condensation, A=2.53 x 108 kPa, B = 5.42 x 103 K. Over ice: L = latent heat of sublimation, A=3.41 x 109 kPa, B = 6.13 x 103 K. Platnick B T p R/Cp Pseudoadiabatic Chart (Stuve diagram) dry adiabat saturation adiabat saturation mixing ratio PHYS 622 - Clouds, spring ‘04, lect.2, Platnick p R/Cp Pseudoadiabatic Chart (Stuve diagram) ex. Parcel at T=30C, 1000mb, w=5 g/kg dry adiabat saturation adiabat saturation mixing ratio PHYS 622 - Clouds, spring ‘04, lect.2, Platnick p R/Cp Pseudoadiabatic Chart (Stuve diagram) ex. Parcel at T=30C, 1000mb, w=5 g/kg dry adiabat LCL ≈ 670mb saturation adiabat saturation mixing ratio PHYS 622 - Clouds, spring ‘04, lect.2, Platnick p R/Cp Pseudoadiabatic Chart (Stuve diagram) ex. Parcel at T=30C, 1000mb, w=5 g/kg 2 g/kg dry adiabat saturation adiabat saturation mixing ratio Platnick p R/Cp Pseudoadiabatic Chart (Stuve diagram) ex. Parcel at T=30C, 1000mb, w=5 g/kg 2 g/kg => parcel water content at 500mb = 3 g/kg dry adiabat saturation adiabat saturation mixing ratio Platnick p R/Cp Pseudoadiabatic Chart (Stuve diagram) ex. Parcel at T=30C, 1000mb, w=5 g/kg LWC = 3 g/kg * rd(T,p) = 3 g/kg * (p/RdT) = 3 g/kg * 0.68 kg/m3 ≈ 2 g/m3 dry adiabat saturation adiabat saturation mixing ratio Platnick Convective development (mesoscale, local) Synoptic development Cold front - steep frontal slopes Warm front - shallow frontal slopes PHYS 622 - Clouds, spring ‘04, lect. 1, Platnick Clouds are difficult, in part, by the nature of the relevant spatial scales and interdisciplinary fields Scale Relevant Physics synoptic ~1000s km (large scale dynamics/thermodynamics, vapor fields) mesoscale ~100s km local (cloud scale) <1-10 km (dynamics/thermodynamics, turbulence, mixing) particle µm - mm (nucleation, surface effects, coagulation, turbulence, stat-mech) molecular PHYS 622 - Clouds, spring ‘04, lect. 1, Platnick Convective condensation level (CCL) • Text book P47 • The convective condensation level (CCL) represents the height where an air parcel becomes saturated when lifted adiabatically to achieve buoyant ascent. It marks where cloud base begins when air is heated from below to the convective temperature, without mechanical lift. CCL vs LCL • The convective temperature (CT or Tc) is the approximate temperature that air near the surface must reach for cloud formation without mechanical lift. In such case, cloud base begins at the convective condensation level (CCL), whilst with mechanical lifting, condensation begins at the lifted condensation level (LCL). Convective temperature is important to forecasting thunderstorm development. • LCL and CCL often agree closely with one another (R&Y, p48) Convection: elementary parcel theory • See Text book p48-50 • Convection can be induced by buoyant or mechanical forces • Buoyant convection represents a conversion of potential energy to kinetic energy. • Velocity can be determined from eq. of motion (4.14) METR215 Clouds Emphasis on cloud microphysics: cloud particle nucleation, growth • Water Clouds – Formation concepts – Water path for adiabatic cloud parcel – Nucleation theory for water droplets (In October) • Ice Clouds • Aerosol-cloud interaction (Martin Leach + Guest Lecture) • Precipitation mechanisms • Cloud Modeling (Guest lecture) PHYS 622 - Clouds, spring ‘04, lect. 1, Platnick