Document
advertisement
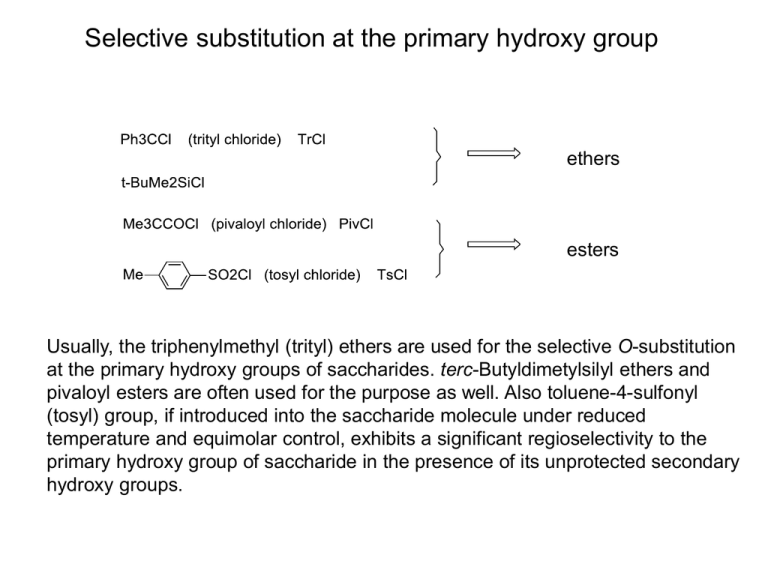
Selective substitution at the primary hydroxy group ethers esters Usually, the triphenylmethyl (trityl) ethers are used for the selective O-substitution at the primary hydroxy groups of saccharides. terc-Butyldimetylsilyl ethers and pivaloyl esters are often used for the purpose as well. Also toluene-4-sulfonyl (tosyl) group, if introduced into the saccharide molecule under reduced temperature and equimolar control, exhibits a significant regioselectivity to the primary hydroxy group of saccharide in the presence of its unprotected secondary hydroxy groups. OTs OH OH O 1 mol TsCl, C5 H5 N OMe OH HO 0 ºC, pyridine OH O OMe OH HO I NaI, Me2 CO reflux, 4 h OH HO 80 % methyl α-Dglucopyranoside O OMe OH 95 % By treatment of methyl α-D-glucopyranoside with one mole of tosyl chloride at reduced temperature, up to 80 % of its 6-O-tosyl derivative can be obtained. This can be then transformed by replacement of its 6-O-tosyl group to different useful 6-substituted D-glucopyranosides, e.g. 6-deoxy-6-iodo derivative (nonanomeric halides of saccharides), but also to 6-amino-6-deoxy derivative (amino saccharides) or 6-deoxy derivative (deoxy saccharides). OTs NH2 OH HO O OMe OH NH3, EtOH OH HO H2, Pd/C O OMe OH N3 NaN3, DMF OH HO O OMe OH Substitutions in situ Ph3 P + EY _ -Y _ +Y OH O OMe OH 18 h, r.t. HO OH ROH - EH + ROP Ph3 Z RZ + Ph3 P=O _ + Ph3 P CX3 X Ph3 P + CX4 OH + Ph3 P E _ _ + OP Ph3 X O OMe HO OH X OH O OMe X = Cl, Br, I HO OH almost quantitative yields at the primary OH group (CH3)2C O CH2 (CH3)2C O O O CH2 O O i Cl O HO O O C(CH3)2 O C(CH3)2 85 % (CH3)2 C O CH2 O O OH i O O CH2Cl O O O (CH3)2C C(CH3)2 O O C(CH3)2 79 % i: TPP, CCl4, reflux, 96 h These replacements can be done under convenient steric conditions also at secondary hydroxy groups, e.g. at the free hydroxy group of 1,2;5,6-di-Oisopropylidene--D-allofuranose. 1,2;5,6-Di-O-isopropylidene--D-glucofuranose does not fulfil the precondition and gives a rearranged product. Amino sugars • Sugar derivatives with one or more OH groups at their carbon skeletone (except of hemiacetal OH group glycosylamines) replaced by a free or substituted amino group. OH OH OH O O HO OH HO NHAc N-acetyl-D-glucosamine (GlcNAc, 2-acetamido-2-deoxy-D-glucopyranose), chitosamine • OH OH NH2 D-glucosamine (GlcN, 2-amino-2-deoxy-D-glucopyranose), chitose Aminosacharidy sa hojne vyskytujú v prírode. N-acetyl-D-glukózamín (chitózamín) je stavebnou jednotkou polysacharidu chitinu alebo jednou zo zložiek polysacharidov kyseliny hyalurónovej a heparínu a tiež mliečnych trisacharidov a vyšších oligosacharidov. N-acetyl-Dgalaktózamín (chondrózamín) je zložkou polysacharidov chondroitín a dermatán. Amino sugars • Sugar derivatives with one or more OH groups at their carbon skeletone (except of hemiacetal OH group glycosylamines) replaced by a free or substituted amino group. OH OH OH O OH HO O NH2 HO NHAc N-acetyl-D-glucosamine (GlcNAc, 2-acetamido-2-deoxy-D-glucopyranose), chitosamine • OH OH -D-glucopyranosylamine (glycosylamine) Aminosacharidy sa hojne vyskytujú v prírode. N-acetyl-D-glukózamín (chitózamín) je stavebnou jednotkou polysacharidu chitinu alebo jednou zo zložiek polysacharidov kyseliny hyalurónovej a heparínu a tiež mliečnych trisacharidov a vyšších oligosacharidov. N-acetyl-Dgalaktózamín (chondrózamín) je zložkou polysacharidov chondroitín a dermatán. Amino sugars • The replacement of an alcoholic hydroxy group of a monosaccharide or monosaccharide derivative by an amino group is envisaged as substitution of the appropriate hydrogen atom of the corresponding deoxy monosaccharide by the amino group. The stereochemistry at the carbon atom carrying the amino group is expressed by regarding the amino group equivalent to OH. OH OH OH HO O OH OH O OH HO NHAc N-acetyl-D-glucosamine (GlcNAc, 2-acetamido-2-deoxy-D-glucopyranose), chitosamine • NHAc N-acetyl-D-galactosamine (GalNAc, 2-acetamido-2-deoxy-D-galactopyranose), chondrosamine 2-Amino-2-deoxysaccharides are abundant in Nature, especially in polysaccharides. N-acetyl-D-glucosamine (chitosamine) is structural unit of polysaccharide chitin, or one of structural units of polysacharides hyaluronic acid and heparín. They also occur in milk trisaccharides and higher oligosaccharides. N-acetyl-D-galactosamine (chondrosamine) is a component of polysaccharides chondroitin and dermatan. Chitin (so-called animal cellulose) is main component of exoskeleton of arthropods Chitinous exoskeleton of insects (a cikada) Zdroj: http://academic.brooklyn.cuny.edu/biology/bio4fv/page/struct-carbohydrates.html http://en.wikipedia.org/wiki/Chitin It is the main component of the cell walls of fungi, the exoskeletons of arthropods such as crustaceans (e.g., crabs, lobsters and shrimps) and insects, the radulas of mollusks, and the beaks and internal shells of cephalopods, including squid and octopuses. Bird plumage and butterfly wing scales are often organized into stacks of nano-layers or nano-sticks made of chitin nanocrystals that produce various iridescent colors by thin-film interference. Supramolecular structure of cellulose n n n http://chemphys.gcsu.edu Amino saccharides * OH O O HO NHAc HO O NHAc O n * O OH Macromolecule of polysaccharide chitin (poly-(-14-D-GlcNAcp)) * OH O O HO OH OH HO O O n * O OH Macromolecule of polysaccharide cellulose (poly-(-14-D-Glcp)) Molecular structure of chitin is very similar to that of cellulose; chitin contains N-acetyl-D-glucosamine monosaccharide units, cellulose is built up of D-glucose monosaccharide units. These structural units of both polysaccharides are linked to each other by β-(1-4)-glycosidic bonds. Supramolecular structure of cellulose http://chemphys.gcsu.edu In addition to basic linear -(14) glycosidic bonds, both intra- and intermolecular hydrogen bonding occurs in cellulose. Structural roles of plant polysaccharides http://academic.brooklyn.cuny.edu/biology/bio4fv/page/struct-carbohydrates.html Amino saccharides * OH O O HO NHAc HO O NHAc O n * O OH Macromolecule of polysaccharide chitin (poly-(-14-D-GlcNAcp)) * OH O O HO OH OH HO O O n * O OH Macromolecule of polysaccharide cellulose (poly-(-14-D-Glcp)) Molecular structure of chitin is very similar to that of cellulose; chitin contains N-acetyl-D-glucosamine monosaccharide units, cellulose is built up of D-glucose monosaccharide units. These structural units of both polysaccharides are linked to each other by β-(1-4)-glycosidic bonds. Amino saccharides HOOC * OH O O O HO O O HO n * NHAc OH Macromolecule of polysaccharide hyaluronic acid (poly-(4-D-GlcAp--13-D-GlcNAcp--1)) HO HOOC * OSO3H O O O O O HO n OH NHAc Macromolecule of polysaccharide chondroitin sulfate (poly-[4-D-GlcAp--13-D-GalNAc(6-OSO3H)p--1]) * Amino saccharides HO3SO * O O O OH O O HO HOOC n * NHAc OH Macromolecule of dermatan-sulfate (chondroitin sulfate B) (poly-[4-L-IdoAp--13-D-GalNAc(6-OSO3H)p--1]) HOOC O O OSO3 H O HO O O HO OH NHSO3 H A part of the heparin macromolecule Structural motifs of the heparin macromolecule GlcA-GlcNAc GlcA-GlcNS IdoA-GlcNS IdoA(2S)-GlcNS IdoA-GlcNS(6S) IdoA(2S)-GlcNS(6S) Heparin Native heparin is a polymer with a molecular weight ranging from 3 kDa to 50 kDa, although the average molecular weight of most commercial heparin preparations is in the range of 12 kDa to 15 kDa. Heparin is a member of the glycosaminoglycan family of carbohydrates (which includes the closelyrelated molecule heparan sulfate) and consists of a variably-sulfated repeating disaccharide unit.[5] The main disaccharide units that occur in heparin are shown below. The most common disaccharide unit is composed of a 2-O-sulfated iduronic acid and 6-O-sulfated, N-sulfated glucosamine, IdoA(2S)-GlcNS(6S). For example, this makes up 85% of heparins from beef lung and about 75% of those from porcine intestinal mucosa.[6] Not shown below are the rare disaccharides containing a 3-O-sulfated glucosamine (GlcNS(3S,6S)) or a free amine group (GlcNH3+). Under physiological conditions, the ester and amide sulfate groups are deprotonated and attract positively-charged counterions to form a heparin salt. It is in this form that heparin is usually administered as an anticoagulant. http://en.wikipedia.org/wiki/Heparin Hyaluronic acid (hyaluronan) Hyaluronan (also called hyaluronic acid or hyaluronate or HA) is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycans in that it is nonsulfated, forms in the plasma membrane instead of the Golgi, and can be very large, with its molecular weight often reaching the millions.[2] One of the chief components of the extracellular matrix, hyaluronan contributes significantly to cell proliferation and migration, and may also be involved in the progression of some malignant tumors. The average 70 kg (154 lbs) person has roughly 15 grams of hyaluronan in the body, one-third of which is turned over (degraded and synthesized) every day.[3] Hyaluronic acid is also a component of the group A streptococcal extracellular capsule,[4] and is believed to play a role in virulence.[5][6] Until the late 1970s, hyaluronan was described as a "goo" molecule, a ubiquitous carbohydrate polymer that is part of the extracellular matrix.[14] For example, hyaluronan is a major component of the synovial fluid, and was found to increase the viscosity of the fluid. Along with lubricin, it is one of the fluid's main lubricating components. http://en.wikipedia.org/wiki/Hyaluronan Hyaluronic acid (hyaluronan), contd. Hyaluronan is an important component of articular cartilage, where it is present as a coat around each cell (chondrocyte). When aggrecan monomers bind to hyaluronan in the presence of link protein, large, highly negatively charged aggregates form. These aggregates imbibe water and are responsible for the resilience of cartilage (its resistance to compression). The molecular weight (size) of hyaluronan in cartilage decreases with age, but the amount increases.[15] Hyaluronan is also a major component of skin, where it is involved in tissue repair. When skin is exposed to excessive UVB rays, it becomes inflamed (sunburn) and the cells in the dermis stop producing as much hyaluronan, and increase the rate of its degradation. Hyaluronan degradation products then accumulate in the skin after UV exposure.[16] While it is abundant in extracellular matrices, hyaluronan also contributes to tissue hydrodynamics, movement and proliferation of cells, and participates in a number of cell surface receptor interactions, notably those including its primary receptors, CD44 and RHAMM. Upregulation of CD44 itself is widely accepted as a marker of cell activation in lymphocytes. Hyaluronan's contribution to tumor growth may be due to its interaction with CD44. Receptor CD44 participates in cell adhesion interactions required by tumor cells. http://en.wikipedia.org/wiki/Hyaluronan Chondroitin sulfate Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage and provides much of its resistance to compression.[1] Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis. Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and Nacetyl-D-galactosamine (GalNAc). Some GlcA residues are epimerized into Liduronic acid (IdoA); the resulting disaccharide is then referred to as dermatan sulfate. As part of aggrecan, chondroitin sulfate is a major component of cartilage. The tightly packed and highly charged sulfate groups of chondroitin sulfate generate electrostatic repulsion that provides much of the resistance of cartilage to compression. Loss of chondroitin sulfate from the cartilage is a major cause of osteoarthritis. http://cs.wikipedia.org/wiki/Chondroitin_sulf%C3%A1t Amino sugars 3-amino-3-deoxy-D-glucose (syn. kanosamine) is a constituent of antibiotics kanamycin and hikizimycin. O HO -Anomer as N-acetyl derivative has m.p. 202 0C and H2 N []D +17 +46 (equilib., water). It can be obtained OH OH e.g. from 1,2;5,6-di-O-isopropylidene-3-O-(toluene-4sulfonyl)--D-allofuranose by treatment with NaN3 in DMF, followed by catalytic hydrogenation of 3-azido-3-deoxy derivative and acid hydrolysis (removal of isopropylidene groups). HOCH2 5-amino-5-deoxy- D-glucose (syn. nojirimycin), the H antibiotic produced by the Streptomyces genus. N HO -Anomer, m.p. 126 0C, []D +100 +73 (equilib., HO water). It can be obtained e.g. via 5-keto- and 5OH OH oxiimino derivative of 3-O-benzyl-1,2-O-isopropylidene6-O-triphenylmethyl--D-glucofuranose. The last step, the acid hydrolysis of 5-amino-5-deoxy-1,2-O-isopropylidene--Dglucofuranose is done with sulfurous acid and gived a stable bisulfite aduct, from which nojirimycin is liberated with an anion exchange resin in the hydroxide form. HOCH2 Amino sugars The important property of 4-amino-4-deoxy- and 5-amino-5-deoxyaldoses is, that their nitrogen atom, being a stronger nucleophile than oxygen of the hydroxy group, participates in forming the respective furanose ring (more precisely, the pyrrolidine ring; 4-amino-4-deoxyfuranose), or the pyranose ring (more precisely, the piperidine ring; 5-amino-5-deoxypyranose). While L-xylose under the thermodynamic equilibrium preferentially forms the six-membered ring, 4-amino-4-deoxy-L-xylose preferentially forms the five-membered ring . H CH=O HO N HO OH CH=O OH HO H2 N OH 4-amino-4-deoxyL-xylose HO HO OH OH HO O HO OH OH 4-amino-4-deoxyL-xylofuranose L-xylose L-xylopyranose OH Amino sugars If the acetamido group instead of the amino group is linked in the C-4 position to the carbonyl group of an aldose, its weaker nucleophilicity (compared to that of the amino group) is not sufficient for the enforcement of the furanose (five-membered) ring and the acetamido sugar usually and preferentially forms the pyranose (six-membered) structure. CH=O AcHN HO OH AcHN O HO OH OH OH 4-acetamido-4-deoxyL-xylopyranose 4-acetamido-4-deoxy-L-xylose Amino sugars A more complicated situation occurs in the case of 5-acetamido-5-deoxy aldoses. These aldose derivatives usually exhibit a more complex thermodynamic equilibrium of both six-membered piperidine forms and five-membered furanose forms. The reason is that the reduced nucleophilicity of the acetamido group is unable to hold the molecule exclusively in the six-membered ring form even though the ring closure through oxygen produces the less common furanose ring modification. CH=O AcHN + H3O O OH (H2SO3) O O Me Me Ac OH OH HO OH NHAc 5-acetamido-5deoxy-D-xylose AcHN N OH HO + O OH OH OH OH dvaparts diely two one part jeden diel 5-acetamido-5-deoxy -D-xylopyranose 5-acetamido-5-deoxy -D-xylofuranose Amino sugars 4-Amino-4-deoxy- and 5-amino-5-deoxy sugars are stable in alkaline solutions. In acidic solutions they rapidly undergo dehydration producing the respective substituted pyrroles a pyridines. HO HO H N OH 5-amino-5-deoxyD-xylopyranose + H N OH OH The reason is the production of stable planar aromatic systems, which are thermodynamically more stable than non-planar six-membered or five-membered rings containing all the sp3 atoms. Deoxy sugars • Derivatives of monosaccharides with one or more hydroxy groups (except of the hemiacetal OH group) replaced with hydrogen atom. Thus they contain in their carbon chain the methylene groups -CH2- (replacement of a secondary OH group) or terminal methyl groups CH3 (replacement of a primary OH group). • If thus the hemiacetal OH group of the cyclic form of aldose is replaced, such derivatives are anhydro alditols. Deoxy sugars O OH HO OH OH H3 C HO O HO H3C HO OH O OH OH OH 2-Deoxy-D-ribose (2-deoxy-D-erythro-pentose) occurs in all cells as a component of deoxyribonucleic acid. L-Rhamnose (6-deoxy-L-manno-hexose) occurs in bacterial and plant polysaccharides and plant glycosides. Glycoside quercitrin is a constituent of the dye quercitron, from which L-rhamnose is isolated after acid hydrolysis. It can be found in oaks species like the North American white oak and European red oak. L-Fucose (6-deoxy-L-galacto-hexose) occurs in plants, animals and microorganisms. It is a constituent of different polysaccharides, e.g. natural gums, and also of maternal milk oligosaccharides and blood group glycoproteins. Most often obtained by acid hydrolysis of brown algae fucan. Blood group antigens O (or 0) antigen A antigen B antigen