Jan 9, 2015. MEMBRANE POTENTIAL
advertisement

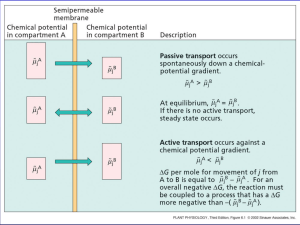
Cellular Neuroscience (207) Ian Parker Lecture # 3 - Membrane potential; Nernst, Goldman equations (How does the selective diffusion of ions across a membrane generate a voltage) http://parkerlab.bio.uci.edu Diffusion The random movements of particles (atoms, ions, molecules or larger) in solution due to thermal energy. Each particle describes a ‘random walk’ , independent (at least for dilute solutions) of other particles. e.g. Go to http://math.furman.edu~dcs/java/rw.html for an applet illustrating a 2-dimensional random walk Everything is random – you can’t predict what any particular molecule will do; BUT, on average, the mean displacement from origin increases as square root of time. Diffusion is responsible for the net movement of a substance down its concentration gradient. There is no ‘driving force’ on any individual molecule, but this is still a useful concept for net movement of many molecules (increasing entropy). Example; diffusion of a dye in water Because diffusion varies is the square root of time, it is very fast over short distances, but very slow over long distances. For a freely-diffusing molecule in water, mean time t to diffuse a distance x; x= t= 1mm 250ms 10mm 25ms 100mm 2.5s 1mm 4min 1cm 6.5hr If our nerves worked by diffusion, not electrically, how long would it take to wiggle your big toe? Diffusion potentials At the first instant solutions are added, K+ ions will move down their concentration gradient. Cl- ions cannot move down their gradient, since the membrane is ipmermeable to ClThus, movement of K+ without corresponding movement of Cl- will set up a potential difference. But, this potential difference will exert a force to stop net movement of further K+ ions (opposite charges attract, like charges repel). Equilibrium potential K+ ions come into equilibrium when the chemical driving force (diffusion down the concentration gradient) equals the electrical driving force. What is the relation between concentration difference and the resulting diffusion potential? i.e. at what voltage is equilibrium reached? Nernst equation E varies logarithmically with concentration ratio E varies linearly with absolute temperature E varies inversely with valence of ion At room temperature and for a monovalent ion; E = 58 mV * log10([K+]in/[K+]out) Easiest to use the Nernst equation to figure out the absolute value of the voltage, and then use common sense to figure out the sign (polarity) Quiz. What are the Nernst potentials for each of these cases? How many ions need to move across the membrane to establish an equilibrium potential? Only enough to charge the membrane capacitance Example, consider a 1 x 1 cm square of lipid membrane, with 1 ml of 0.1.M KCl on one side and 1 ml of 1 M KCl on the other. E = 58 mV C = 1 mF (remember specific capacitance of membrane ~ 1 mF/cm2) Charge q = C*V = 10-6 * 5.8 x 10-2 = 5.8 x 10-8 Coulombs Faraday’s constant ~ 105 coulombs/mole So, about 5.8 x 10-13 moles of K+ ions would need to move to establish the equilibrium potential. We started with 10-3 moles of K+ (I M in 1 ml) Take-home message – The number of ions that move to establish an equilibrium potential is vanishingly small. Concentrations of ions on either side of the membrane remain almost constant, and the concentrations of cations (+) and anions (-) are ALMOST equal on each given side of the membrane. What about real cells? For frog muscle;; Resting potential is ~ -80 mV Thus, K+ and Cl- ions are close to equilibrium, but Na+ and Ca2+ are far from equilibrium. What ion(s) determine the resting potential? Can find out by changing the extracellular concentration of different ions and measuring effect on resting potential Membrane potential of frog muscle behaves like a K+ electrode – Nernstian relationship with [K+] out, except for deviation at very low [K+], owing to small permeability to Na+. Changing the concentration of other ions has very little effect (i.e. membrane has low permeability at rest to Na+, Ca2+ and Cl-) How can we deal with membranes that are permeable to more than one ion? The Goldman-Hodgkin-Katz equation (AKA Goldman, or constant-field equation) This is just an expanded form of the Nernst equation, with the different ions weighted by their permeabilities. Eg. if PK >> PNa PNa >> PK PK = PNa E ~ -101 mV (EK) E ~ +59 mV (ENa) E ~ -21 mV (mid-way between EK and ENa) In practice, it is easier to measure the relative permeabilities of ions, rather than their absolute permeabilities. So, re-write the Goldman equation in a more useful form; b = PNa/PK, c = PCl/PK (Note, concentrations for Cl- are inverted, to account for difference in sign) The permeability of nerve membrane to various ions (and hence the membrane potential) is determined by the opening of ion channels, which may be regulated by voltage, extracellular ligands, intracellular second messengers etc…