Differential Rate Laws
advertisement
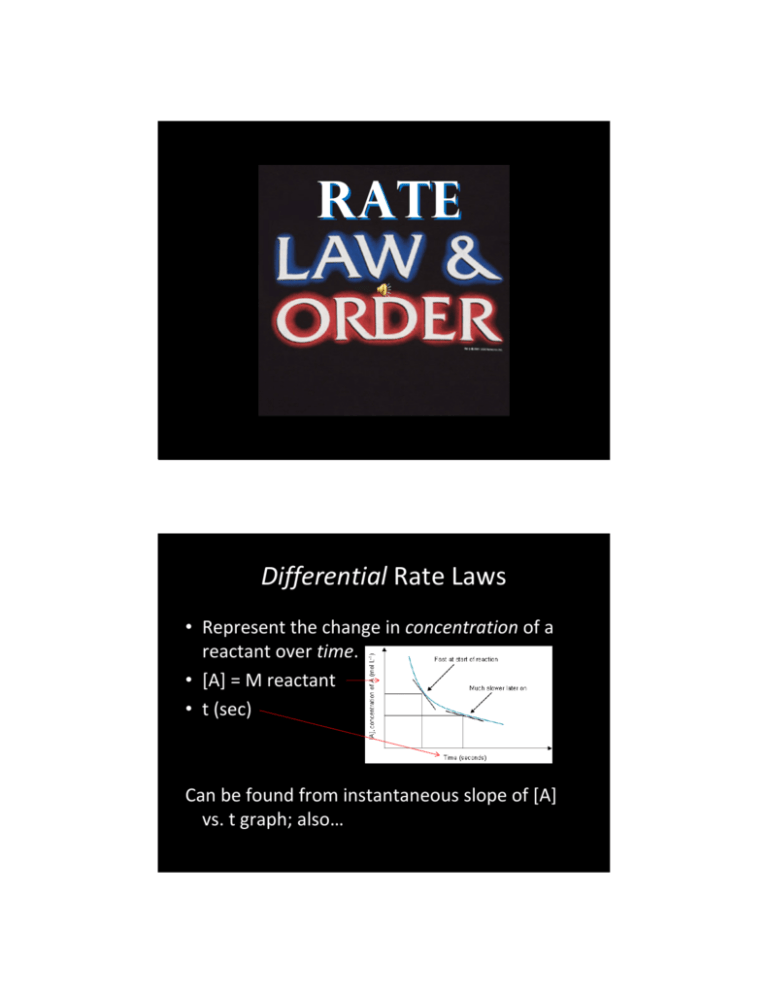
RATE Differential Rate Laws • Represent the change in concentration of a reactant over time. • [A] = M reactant • t (sec) Can be found from instantaneous slope of [A] vs. t graph; also… Experimental Data! An experiment measuring the concentration of N2O5 is conducted: Time (min) [N2O5] (M) 0 0.1756 20 0.0093 40 0.00531 60 0.00295 80 0.00167 100 0.00094 160 0.00014 rate = Δ[A ] (.0093M − .1756M ) M = = −0.00832 Δt (20 min − 0 min) min •Rate is calculated using the standard Δy/Δx formula. •The rate is negative because N2O5 is being consumed. Experimental Data! An experiment measuring the concentration of N2O5 is conducted: Time (min) [N2O5] (M) d[N2O5]/dt 0 0.1756 ‐0.008315 20 0.0093 ‐0.0001995 40 0.00531 ‐0.000118 60 0.00295 ‐0.000064 80 0.00167 ‐0.0000365 100 0.00094 ‐1.333E‐05 160 0.00014 •Rate is calculated using the standard Δy/Δx formula. •The rate is negative because N2O5 is being consumed. •The rate is not constant! •Why do you think this is? Stoichiometry and Rate • Since rates are measured using moles, relate rates by stoichiometry: 2N2O5 Æ 4 NO2 + O2 rate = - Δ[N 2 O 5 ] dt ⎛ Δ[N 2 O 5 ] ⎞⎛ 1 mol O 2 O 2 rate = ⎜ ⎟⎜⎜ dt ⎝ ⎠⎝ 2 molN 2 O 5 ⎞ 1 ⎟⎟ = rate N 2 O 5 ⎠ 2 •the sign for the rate changes as you cross the arrow (rate of appearance is the opposite of the rate of disappearance) •Simply use the mole ratio to express rates in terms of another reactant or product. The Rate Law Expression • For any reaction, we only care about reactants: A + 2B Æ C + D • We can derive a general expression for the rate, called the rate law: Rate = k[A]m[B]n • k is the rate constant. Its units vary, depending on the rest of the equation. • [A] and [B] are concentrations. At this point, the stoichiometry of the original reaction is irrelevant – ignore it. • m and n are the orders of the reaction. – Order means the same thing here as it does in algebra (first order, second order polynomial—the degree (exponent) of x). – Here they are the dependence of the rate on that reactant. Solving the Rate Law Expression, I Find the order of the reaction from given data: a. Write the general rate law expression: Rate = k[A]m[B]n b. Solve for m by choosing two trials where B is constant (like 1 and 2) m ⎛ rate trial 1 ⎞ ⎛ [A]trial 1 ⎞ ⎜⎜ ⎟⎟ = ⎜⎜ ⎟⎟ ⎝ rate trial 2 ⎠ ⎝ [A]trial 2 ⎠ m m ⎛ .002 ⎞ ⎛ 0.5 ⎞ ⎛1⎞ ⎛1⎞ ⎟ ⇒⎜ ⎟=⎜ ⎟ ⇒m=2 ⎜ ⎟=⎜ ⎝ .008 ⎠ ⎝ 1 ⎠ ⎝4⎠ ⎝2⎠ Orders on the AP exam are almost always 0, 1, or 2. Sometimes (2009) they are non‐ integral, so TRUST YOUR MATH! Solving the Rate Law Expression, II • Keep finding the order using the same method for each reactant. • n is the order for B • n = 0 • A doubles, but no change in rate (b does not affect the rate!) Once you have found the orders, write the general rate law expression: rate = k[A]2 Then… [B] is not included because it would be [B]0 Solving the Rate Law Expression, III • Use data in the table to find k. Choose any trial you want, because k is a CONSTANT! • Plug and chug: rate = k[A ] 2 mol mol .002 .002 rate L s ⋅ L ⋅ s = .008 L ⋅ mol = k= = 2 s ⎛ [A]2 ⎛ mol 2 ⎞ mol ⎞ ⎜ 0.25 2 ⎟ ⎜ 0.5 ⎟ ⎟ ⎜ L L ⎠ ⎝ ⎠ ⎝ Once you have found the rate constant, write the specific rate law expression: rate = .008[A]2 Integrated Rate Laws • Differential rate laws look at differing rates with different concentrations • It is a lot easier to collect time based rate data: Time (min) [N2O5] (M) d[N2O5]/dt 0 0.1756 ‐0.008315 20 0.0093 ‐0.0001995 40 0.00531 ‐0.000118 60 0.00295 ‐0.000064 80 0.00167 ‐0.0000365 100 0.00094 ‐1.333E‐05 160 0.00014 • But how do you turn this into a rate law expression? • The magic of calculus… Why they call them Integrated rate laws First Order Reactions rate = k[ A] = − d [ A] dt d [ A] [ A] d [ A] − kdt = [ A] − kt + C = ln[ A] + C ln[ A] = −kt + ln[ A]0 − kdt = ∫ ∫ Second Order Reactions Rate laws rate = k[ A]2 = − rearrange − kdt = Integrate! ∫ Combine the Cs d [ A] dt d [ A] [ A]2 d [ A] − kdt = [ A]2 1 − kt + C = − +C [ A] 1 1 = kt + [ A] [ A] 0 ∫ Constants are concentrations, [A]0 is the original concentration at t=0; [A] the concentration at time t. Rate Laws and Lines First order: ln [A] = ‐kt + ln[A]0 •If a plot of ln[A] vs. t gives a straight line, then the reaction is first order • Slope = ‐k Second order: 1/[A] = kt + 1/[A]0 •If a plot of 1/[A] vs. t gives a straight line, then the reaction is second order • Slope = k Half‐Life How do you solve half‐life problems? But seriously… Half‐Life = length of time for a chemical concentration to decrease by half. Formulas: 1st order: t1/2 = .693/k 2nd order: t1/2 = 1/(k[A]0) Where k= rate constant. Also, you can brute‐force it: [A]/[A]0 = (1/2)n Where n is the number of half‐lives The Arrhenius Equation (it’s the Arrheniest!) 1. 2. 3. 4. k is k, our friend the rate constant. A is the frequency factor EA is the activation energy R and T are the thermodynamic gas constant R and absolute temperature, T k= -E A Ae RT The real value of the A.E. is plotting ln k vs. T: The slope of the line is –EA/R, giving you the activation energy for the reaction! Mechanisms Mechanisms are the actual means by which reactions occur. The foundational thoughts in proposing mechanisms are 1. Reactions can only happen when a collision occurs between two species. They will not necessarily react, but they don’t have a chance without a collision. 2. Collisions must occur between two species only (“bimolecular”). The more species you involve in a simultaneous collision the less likely it is to occur. 3. Mechanisms can have many individual steps, but these must add up to the complete, balanced, observed overall reaction. 4. There may be many intermediate species (called intermediates) which may come and go throughout the reaction, but they are not listed as reactants or products. 5. These steps will occur at different rates, but the slowest overall step determines the overall rate of the reaction. This is called the rate determining step. Let’s propose a mechanism! The observed reaction is 2NO2 + F2 Æ 2NO2F • The following mechanism is proposed: NO2 + F2 Æ NO2F + F NO2 + F Æ NO2F 2NO2 + F2 Æ 2NO2F •Steps add up to overall reaction •Each step is biomolecular The observed rate law is Rate = k[NO2][F2] What about the kinetics? The mechanism works if the slow step has stoichiometry that agrees with the orders shown in the observed rate law. •First order in NO2 •First order in F2 •First step stoichiometric coefficients are all 1, so it agrees! What if the first step isn’t slow? • The rate determining step must still agree with the rate law. This takes creativity: Reaction: 2NO + O2 Æ 2NO2 •Steps add up to overall reaction •Each step is biomolecular, but… Mechanism: It gives us this rate law: 2NO N2O2 + O2 Ý Æ N2O2 (fast) 2NO2 (slow) 2NO + O2 Æ 2NO2 Rate = k[N2O2][O2] The observed rate law is Rate = k[NO]2[O2] What do we do to fix this? The Magic of Algebra The key is this equilibrium: 2NO Ý N2O2 (fast) At equilibrium, the forward and reverse reaction rates are equal: rate forward = k forward [ NO]2 = ratereverse = k reverse [ N 2 O 2 ] k forward [ NO]2 = k reverse [ N 2 O 2 ] k forward k reverse [ NO]2 = [ N 2 O 2 ] Our slow step is N2O2 + O2 Æ 2NO2 (slow) If we substitute using the rearrangement above, then our new rate law is Rate = k (kf/kr)[NO]2[O2] if we combine all the k’s, we get… Rate = k[NO]2[O2] Mechanism Summary 1. Look at the proposed mechanism 1. 2. Check to see if steps are all bimolecular Check to see if steps add up to observed reaction 2. Check to see if the first step is slow or fast. 1. Slow 1. 2. 2. See if stoichometric coefficients of the reactants are the same as the order of the reactants in the observed rate law If so, the mechanism works Fast 1. 2. Do the rearrangement and see if you can make the slow step agree with the observed rate law If so, the mechanism works. Every AP Exam Kinetics Question 1. 2. 3. 4. 5. 6. Find the orders. Find k (with units) Write the specific rate law Find a rate given data Does this mechanism work? Determine order from a plot of ln[A] or 1/[A] vs. t 7. Use the Arrhenius equation to find k or Ea