Cellular Energetics: Thermodynamics, ATP, Cellular
advertisement
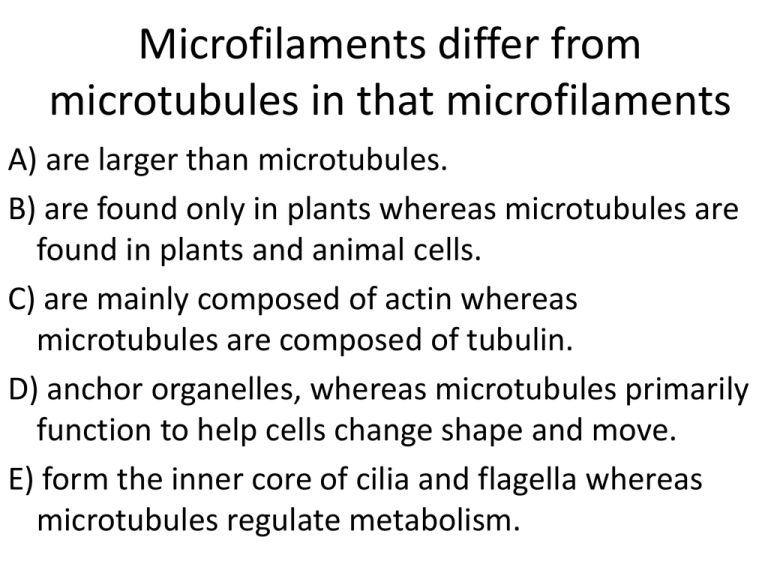
Microfilaments differ from microtubules in that microfilaments A) are larger than microtubules. B) are found only in plants whereas microtubules are found in plants and animal cells. C) are mainly composed of actin whereas microtubules are composed of tubulin. D) anchor organelles, whereas microtubules primarily function to help cells change shape and move. E) form the inner core of cilia and flagella whereas microtubules regulate metabolism. Cellular Energetics: Thermodynamics, ATP, and Enzyme catalysis Campbell Biology Chapter 5 Energy and Thermodynamics Energy is the capacity to do work • There are many forms of energy: – – – – Kinetic energy Potential energy Chemical energy Electrical energy All Living Things Require and Consume Energy • We get our energy from food • Ultimate source of energy for all life on earth is the sun The First Law of Thermodynamics • Energy cannot be created or destroyed • The amount of energy in the universe is constant • Energy can be interconverted from one form to another: – Potential energy – Kinetic energy – Radiant energy Potential energy • Energy is the ability to do work • Potential Energy of position • Gravitational potential energy • Chemical potential energy Kinetic energy • Energy of motion • KE= 1/2mv2 • Temperature is a measure of molecular kinetic energy The 1st Law of Thermodynamics: Energy can be interconverted from one form to another More energy interconversions The 2nd Law of Thermodynamics The Law of Entropy • Interconversions of energy are never 100% efficient • Entropy! • Entropy is a measure of disorder (i.e. chaos, randomness) • Each interconversion of energy involves loss of usable energy : Entropy in Action Biochemical reactions are inefficient The price of minimizing entropy is the constant expenditure of free energy Closed systems will deplete themselves of usable (free) energy • Given a finite amount of energy, each energy interconversion will result in an everincreasing amount of unusable energy (entropy) Recognizing Entropy in the world Which system has more entropy? A B Can living systems reduce entropy? Recognizing Enthalpy B Enthalpy = Energy in chemical bonds Which systems have more Enthalpy? These? Or these? Biochemical reactions are spontaneous only if ∆G is negative • Reactions which release energy are exergonic • Reactions which require energy are endergonic ∆ G = ∆H - T∆S • Only exergonic processes with a negative ∆G are spontaneous • Spontaneous processes can be harnessed to perform work If ΔG < 0, the reaction is spontaneous (it will happen) C6H12O6(s) + 6O2(g) 6CO2(g)+ 6H2O(l) Will the Reaction happen? Well, is heat given off? Does entropy increase? G = ∆H - T∆S ∆H = enthalpy (heat in chemical bonds) ∆S= Degree of entropy (chaos) created by Rxn T= Temperature at which Rxn occurs Important: Spontanous ≠ fast LE 5-2b Heat Chemical reactions Carbon dioxide Glucose ATP ATP Water Oxygen Energy for cellular work Which of these diagrams depicts an endergonic reaction? Reactants Energy required Reactants A Amount of energy required Potential energy of molecules Potential energy of molecules Products Amount of energy released Energy released Products B LE 8-7a G < 0 A closed hydroelectric system G = 0 LE 8-7c G < 0 G < 0 G < 0 A multistep open hydroelectric system In living things, a state of equilibrium most often means ___________. A) B) C) D) E) Efficiency is optimized The reaction is Endothermic Enthalpy is increased Entropy is minimized You are dead ATP A steer must eat over 100 pounds of grain to gain less than 10 pounds of • muscle tissue. This illustrates A) the first law of thermodynamics. B) the second law of thermodynamics. C) that some energy is destroyed in every energy conversion. D) that energy transformations are typically 100% efficient. E) None of the choices are correct. Living cells manage to perform endergonic activities • How is this possible? ATP hydrolysis can be coupled to endergonic reactions to power cellular work • A cell does three main kinds of work: – Mechanical – Transport – Chemical • To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one The Structure and Hydrolysis of ATP • ATP (adenosine triphosphate) is the cell’s energy shuttle • ATP provides energy for cellular functions • ATP is a nucleic acid monomer ATP is the energy currency of all living things Adenine Phosphate groups Ribose ATP: Adenosine Triphosphate LE 8-9 P P P Adenosine triphosphate (ATP) H2O Pi Inorganic phosphate + P P Adenosine diphosphate (ADP) + Energy Phosphorylation can change the conformation of proteins LE 8-10 Anabolic (building up) reactions are usually endergonic NH2 Glu + NH3 Ammonia Glutamic acid G = +3.4 kcal/mol Glu Glutamine Breakdown of ATP is exergonic ATP + H2O ADP + P i G = –7.3 kcal/mol Coupled reactions: Overall G is negative; together, reactions are spontaneous G = –3.9 kcal/mol How ATP Performs Work • ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to some other molecule, such as a reactant • The recipient molecule is now phosphorylated Three types of cellular work are powered by ATP hydrolysis •Mechanical •Transport •Chemical The Regeneration of ATP • ATP is regenerated by addition of a phosphate group to ADP • The energy to phosphorylate ADP comes from food • The chemical potential energy temporarily stored in ATP drives most cellular work LE 8-12 ATP Energy for cellular work (endergonic, energyconsuming processes) Energy from catabolism (exergonic, energyyielding processes) ADP + P i Enzymes At which level of protein structure are interactions between R groups most important? A) primary B) secondary C) tertiary D) quaternary E) the R groups are not related to the overall structure of a protein Sugar is an energy-rich molecule • Breakdown of sugar is spontaneous • C6H12O6(s) + 6O2(g) 6CO2(g)+ 6H2O(l) Wood and paper are made of cellulose • Cellulose is a polymer of glucose • Why doesn’t our jar of sugar burst into flame? Exergonic reactions still require activation energy • Spontaneous ≠ fast • Ea is dependent on temperature • At high temperatures, reactions happen faster Jumping bean analogy • Molecules are like jumping beans • Temperature ≈ height of jump • Living things cannot wait for a good jump • After a long time, where will the beans be? • All of them? • Will they ever stop jumping? Living things can use enzymes to speed up reactions • Enzymes speed up reactions by lowering energy of activation • They are catalysts Catalysts speed up reactions • Platinum is used in catalytic converters • 2CO + 02 2CO2 • Catalysts are not consumed in a reaction • They cannot add energy to a reaction Enzymes are protein catalysts • Catalysts- things added to chemical reactions which speed up those reactions • Catalysts are not consumed in a reaction • Catalysts cannot add energy to a reaction • -ase: The enzyme suffix Catalase Enzymes can dramatically lower the energy of activation for a reaction no enzyme with enzyme Ea Energy Ea reactants products Reaction Course Note that the equilibrium of the reaction is unaffected 12 How enzymes work Structure aids function An active site naturally fits substrate Enzyme specificity depends on shape Substrate Binding and Reaction Some important Enzymes Cellulase ATP synthase Nitrogenase Catalysis in the Enzyme’s Active Site • In an enzymatic reaction, the substrate binds to the active site • The active site can lower an EA barrier by – Orienting substrates correctly – Straining substrate bonds – Providing a favorable microenvironment – Covalently bonding to the substrate LE 5-6 Enzyme available with empty active site Active site Substrate (sucrose) Substrate binds to enzyme with induced fit Glucose Enzyme (sucrase) Fructose H2O Products are released Substrate is converted to products Factors Affecting Enzyme Activity 1. 2. 3. 4. Salts Temperature pH Inhibitors and Activators Effects of Temperature and pH • Each enzyme has an optimal temperature in which it can function • Each enzyme has an optimal pH in which it can function • Tertiary structure can be radically altered by changes in pH LE 8-18 Optimal temperature for typical human enzyme 0 Optimal temperature for enzyme of thermophilic (heat-tolerant bacteria) 40 60 Temperature (°C) 20 80 100 Optimal temperature for two enzymes Optimal pH for pepsin (stomach enzyme) 0 1 2 3 Optimal pH for trypsin (intestinal enzyme) 4 5 pH Optimal pH for two enzymes 6 7 8 9 10 Enzyme Inhibition • Competitive inhibitors bind to the active site of an enzyme, competing with the substrate • Noncompetitive (allosteric) inhibitors bind to another part of an enzyme, causing the enzyme to change shape (allostery) and making the active site less effective Many drugs are enzyme inhibitors • Protease inhibitors fight HIV Can enzymes catalyze endothermic reactions? • If so, how? • If not, why not? Inhibition of an enzyme is irreversible when A) a competitive inhibitor is involved. B) a noncompetitive inhibitor is involved. C) the shape of the enzyme is changed. D) bonds form between inhibitor and enzyme. E) None of the choices are correct.