Chapter 7
advertisement

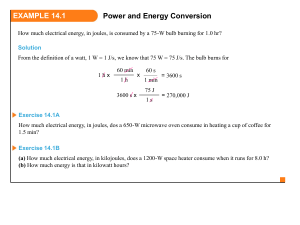
Chapter 7 Thermochemistry 7.1 Terminology System- the part you are investigating Surroundings- the rest of the universe Open System: freely exchanges matter and energy Closed System: exchanges energy but not matter Isolated System: does not interact with surroundings Energy The ability to do work or transfer heat. –Work: Energy used to cause an object that has mass to move. –Heat: Energy used to cause the temperature of an object to rise. 4 Work • Energy used to move an object over some distance. • w = F d, where w is work, F is the force, and d is the distance over which the force is exerted. 5 Kinetic Energy Energy an object possesses by virtue of its motion. 1 KE = mv2 2 Kinetic energy increases as the speed and mass of an object increases. 6 Potential Energy Energy an object possesses by virtue of its position or chemical composition. PE= m x g x h m=mass=kg g=gravitational constant=9.8m/s2 h= height (or distance) 7 7.2 Heat Energy transferred between a system and its surroundings as a result of temperature difference Heat flows from a warm body to a cooler body. The quantity of heat, q, required to change a substances temperature is dependent on: – Temperature change – Quantity of substance – Nature of substance calorie: heat to change 1 g of water 1°C 1 cal = 4.184 J Specific Heat Capacity: heat to change 1 g of a substance 1°C Example 1A How much heat in kJ is required to raise the temperature of 237 g of water from 4.0°C to 37.0°C? q = mCΔT qsystem +qsurroundings = 0 Example 2A • When 1.00 kg of lead (c = 0.13J/g°C) at 100.0°C is added to a quantity of water and the final temperature of the lead/water is 32.5°C. What is the mass of water present? Specific Heat values given on page 247 of textbook. 7.3 Calorimetry Chemical Energy: the energy associated with chemical bonds and intermolecular forces Chemical Reaction: breaking and forming bonds Heat of Reaction: heat exchange during a chemical reaction Exchange of Heat • When heat is absorbed by the system from the surroundings, the process is endothermic. 15 Exchange of Heat • When heat is absorbed by the system from the surroundings, the process is endothermic. • When heat is released by the system to the surroundings, the process is exothermic. 16 Bomb Calorimetry Reactions can be carried out in a sealed “bomb” and measure the heat absorbed by the water. 17 Example 3A • Vanillin in a natural constituent of vanilla. It is also manufactured for use in artificial vanilla flavor. The combustion of 1.013 g of vanillin, C8H8O3, in a bomb calorimeter causes the temperature to raise from 28.89°C to 30.09°C. What is the heat of combustion of vanillin in kJ/mol? Constant Pressure Calorimetry By carrying out a reaction in aqueous solution in a simple calorimeter such as this one, one can indirectly measure the heat change for the system by measuring the heat change for the water in the calorimeter. 19 Example 4A Two solutions, 100.0 mL of 1.00M AgNO3(aq) and 100.0 mL of 1.00M NaCl (aq), both initially at 22.4°C are added to a Styrofoam cup allowed to react. The temperature rises to 30.2°C. Determine the qrxn per mole of AgCl(s) in the reaction Ag+(aq) + Cl-(aq) AgCl(s) 7.4 Work We can measure the work done by the gas if the reaction is done in a vessel that has been fitted with a piston. w = −PV 21 Example 5A How much work, in Joules, is involved when 0.255 mol N2 at constant temperature of 23°C is allowed to expand by 1.50L in volume against an external pressure of 0.750 atm? 7.5 First Law of Thermodynamics • Energy is neither created nor destroyed. • In other words, the total energy of the universe is a constant; if the system loses energy, it must be gained by the surroundings, and vice versa. qsystem = -qsurroundings U = q + w 23 E, q, w, and Their Signs q + (system gains heat) - (system loses heat) w + (work done on system) - ( work done by system) U + (net gain of energy by system) - ( net loss of energy by system) 24 Example 6A In compressing a gas, 355J of work is done on the system. At the same time 185 J of heat escapes from the system. What is the U for the system? 7.6 Enthalpies of Reaction This quantity, H, is called the enthalpy of reaction, or the heat of reaction. 26 Example 7 How much heat is associated with the complete combustion of 1.00kg of sucrose? ΔH = -5.65 x 103 kj/mol Example 7A What mass of sucrose must be burned to produce 1,000 kJ of heat? ΔH = -5.65 x 103 kj/mol Changing States Example 8A What is the enthalpy change when a cube of ice 2.00 cm on edge is brought from -10.0°C to a final temperature of 23.2°C? For ice, use d = 0.917 g/mL, a specific heat of 2.01 J/g°C and enthalpy of fusion of 6.01kJ/mol. 7.7 Hess’s Law Change in enthalpy is the same if the reaction took one step or many steps • If you can add balanced equations, you can add ΔH • If you alter the equation, alter ΔH – Double reactant/products, double ΔH – Reverse equation, change sign of ΔH – Cancel items if they are in reactants and products Hess’s Law Example 7.8 Heat of Formation ΔHf°: enthaply of formation • Defined as heat change that results when one mole of compound is formed from its constituent elements • Pure element ΔHf°= 0 Example 10A The standard enthalpy of formation for the amino acid leucine is -637.3 kJ/mol C6H13O2N (s). Write the chemical equation to which this value applies. Finding ΔHrxn ΔHrxn = Σ Hf°(products) - Σ Hf°(reactants) Values in back of textbook Example 11A Use data table 7-2 to calculate the enthalpy for the combustion of ethanol, CH3CH2OH (l), at 298.15K. Example 12A Determine the standard enthalpy for the formation of glucose C6H12O6. ΔHrxn = 2803 kJ Example 13A Given that Hf°[AgI(s)] = -61.84 kJ/mol, what is the standard enthanpy change for the precipitation of silver iodide? Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) 39 Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) 40 Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • Imagine this as occurring in 3 steps: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) 41 Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) • The sum of these equations is: C3H8 (g) 3 C(graphite) + 4 H2 (g) 3 C(graphite) + 3 O2 (g) 3 CO2 (g) 4 H2 (g) + 2 O2 (g) 4 H2O (l) C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) 42 Calculation of H We can use Hess’s law in this way: H = nHf(products) - mHf(reactants) where n and m are the stoichiometric coefficients. 43 Calculation of H C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (l) H = = = = [3(-393.5 kJ) + 4(-285.8 kJ)] - [1(-103.85 kJ) + 5(0 kJ)] [(-1180.5 kJ) + (-1143.2 kJ)] - [(-103.85 kJ) + (0 kJ)] (-2323.7 kJ) - (-103.85 kJ) -2219.9 kJ 44