Chapter 3: Structure of Metals & Ceramics
advertisement
L03A: Chapter 3
Structures of Metals & Ceramics
• The properties of a material depends on the arrangement of atoms within the
solid.
• In a single crystal the atoms are in an ordered array called the structure.
Single crystals are necessary for many applications and can be very large.
For example, silicon crystals can be up to 2 feet in diameter:
http://www.flickr.com/photos/davemessina/6231300549/
• A polycrystalline material consists of many crystals. Materials used for
construction or fabrication are usually polycrystalline. For example:
http://www.cartech.com/news.aspx?id=578
• In this chapter we examine typical crystal structures for metals, inorganic
compounds, and carbon.
• You will see how to specify crystal planes and directions.
• You will learn how to calculate some properties of crystals from their
structure, including the dependence on direction in their lattice.
• Review calculation of areas for squares and rectangles, and calculation of
distances and areas for right triangles, e.g. at the Math Skills Review under
Read, Study & Practice at WileyPLUS.com.
Last revised January 12, 2014 by W.R. Wilcox, Clarkson University
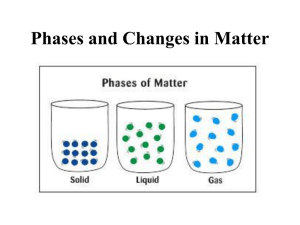
Amorphous and crystalline materials
• A material is crystalline if the atoms display long-range order, i.e. the same
repeating arrangement over-and-over.
• The atoms in some materials do not have long-range order. These are
called amorphous or glassy. Most polymers are amorphous, but so are
some ceramics, metals, and forms of carbon.
crystalline SiO2
amorphous SiO2
• Equilibrium structures are those with the minimum Gibbs energy G, although
atomic movement in solids is so slow that equilibrium is often not reached at
room temperature. (In thermodynamics you’ll see that G = H – TS)
Hard-sphere model of crystals
• We may show the atoms as points or small spheres connected by lines, or
we may show them as hard spheres of defined diameter in contact with one
another.
• For a metal with a face-centered cubic lattice:
Unit cell. When repeated,
generates the entire crystal.
Metallic Crystal Structures
•
•
•
•
•
•
Bonding is not directional
Minimum energy when nearest-neighbor distances are small.
The electron cloud shields the positive cores from one another.
Metals have the simplest crystal structures.
We will examine the three most common.
Two of their unit cells are based on a cube:
Body-centered cubic (BCC)
Face-centered cubic (FCC)
Virtual Materials Science and Engineering (VMSE):
http://higheredbcs.wiley.com/legacy/college/callister/1118061608/vmse/xtalc.htm
Atomic Packing Factor (APF)
Volume of atoms in unit cell*
APF =
Volume of unit cell
* assuming hard spheres
APF calculation for a simple cubic structure
a
R=0.5a
atoms/unit cell = 8 x 1/8 = 1
volume
atoms
atom
4
unit cell
p (0.5a) 3
1
3
APF =
= 0.52
a3
volume
unit cell
The coordination number is the number of nearest neighbors.
What is it here?
Body Centered Cubic Structure (BCC)
Examples: Cr, W, Fe(), Ta, Mo
Click on image for animation
(Courtesy P.M. Anderson)
All atoms are identical and are
colored differently only
for ease of viewing.
Atoms touch only along
cube diagonals.
Coordination number?
How many touch the one in
the center?
Coordination number = 8
Number of atoms per unit cell?
1 center + 8 corners x 1/8 = 2
Atomic Packing Factor for BCC
3a
a
2a
R
a
Close-packed directions:
length = 4R = 3 a
atoms
volume
4
p ( 3a/4) 3
2
unit cell
atom
3
APF =
= 0.68
volume
3
a
unit cell
Theoretical Density
Density = =
=
where
Mass of Atoms in Unit Cell
Total Volume of Unit Cell
nA
VC NA
n = number of atoms/unit cell
A = atomic weight (g/mol)
VC = Volume of unit cell = a3 for cubic
NA = Avogadro constant
= 6.022 x 1023 atoms/mol
8
Example: Theoretical Density of Chromium
• Cr is body-centered cubic
A = 52.00 g/mol
R = 0.125 nm
n = 2 atoms/unit cell
a = 4R/ 3 = 0.2887 nm
R
a
atoms
unit cell
=
volume
2 52.00
a3 6.022 x 1023
unit cell
theoretical = 7.18 g/cm3
experimental = 7.19 g/cm3
g
mol
atoms
mol
Face Centered Cubic Structure (FCC)
Examples: Al, Cu, Au, Pb, Ni, Pt, Ag
Atoms only touch along
face diagonals.
How many atoms in the unit cell
touch the atom in the center of
the front face?
How many additional atoms touch
it in the unit cell in front of this one?
Coordination number = 8 + 4 = 12
How many atoms in one unit cell?
6 face x 1/2 + 8 corners x 1/8 = 4
Atomic Packing Factor for FCC
2a
Close-packed directions:
length = 4R = 2 a
a
atoms
volume
4
3
p ( 2a/4)
4
unit cell
atom
3
APF =
= 0.74
volume
3
a
unit cell
This is the maximum achievable APF and
is one of two close-packed structures.
Crystal Systems
a, b, and c are the lattice constants
Only for the cubic system are
the angles all 90o and the lattice
constants all the same.
Crystal structure
• Seven different possible geometries for the unit cell.
• There are 14 Bravais lattices, with each point representing the same atom or
collection of atoms.
• Pure metals are
usually FCC, BCC or
HCP.
• Except for
hexagonal, number
of atoms per unit cell:
1/8 at corners
1/2 at face centers
All of body centered
Point Coordinates in a Lattice
z
Point coordinates for the unit cell
center are
111
c
a
a/2, b/2, c/2 ½ ½ ½
y
000
x
b
Point coordinates for unit cell
corner are a, b, c 111
·
z
2c
·
·
·
b
b
y
Translation by an integer multiple
of lattice constants reaches an
identical position in another unit
cell
Miller Indices for Crystallographic Directions
Algorithm
z
y
x
1. If necessary, translate the vector so it starts
at the origin.
2. Read off the end of the vector in increments
of unit cell dimensions a, b, and c.
3. Adjust these to the smallest integer values.
4. Enclose in square brackets without commas.
That is, [uvw]
examples: 1, 0, ½ => 2, 0, 1 => [201]
-1, 1, 1 => [ 111 ]
where the overbar represents
a negative index
families of directions <uvw> , for example:
[100],[010],[001],[ 1 00],[0 1 0],[00 1] 100
VMSE with examples, problems, exercises
Linear Density of Atoms (LD)
Number of atoms
LD =
Length of direction vector
example: linear density of Al in [110] direction
FCC with a = 0.405 nm
[110]
# atoms
LD =
length
2
= 3.5 nm-1
2a
a
16
Miller Indices for Crystallographic Planes
• Reciprocals of the three axial intercepts for a plane, cleared of fractions &
common multiples.
• All parallel planes have the same Miller indices.
• Algorithm (procedure):
1. If the plane passes through the origin, translate so it does not.
2. Read off the intercepts of the plane with the axes in increments
of the lattice constants (a, b, c).
For example, 1, 2, 2
3. Take reciprocals of those intercepts. If it is parallel to an axis
so that it doesn’t intersect it, the reciprocal is 0.
For example, 1, ½, ½
4. Convert the numbers to the smallest possible integer values.
For example, 2, 1, 1
5. Enclose those numbers in parentheses, with no commas.
For example (211).
6. As with directions, a bar over a number indicates it is negative.
• VMSE with illustrations, problems, exercises
• Families of equivalent planes. For a cubic structure, for example:
211 (211),(121),(112),(211),(2 1 1),(21 1),etc.
Three Low-index Planes
Crystallographic Plane Examples
z
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1
1/1
1
1
4.
Miller Indices
(110)
example
1. Intercepts
2. Reciprocals
3.
Reduction
a
1/2
1/½
2
2
4.
Miller Indices
(100)
b
1
1/1
1
1
c
1/
0
0
c
y
b
a
x
b
1/
0
0
c
1/
0
0
z
c
y
a
b
x
19
Planar Density or Packing
•
•
•
•
Atoms per unit area
Very important for mechanical strength and for
chemical properties.
Essential step is to sketch the plane of interest, and
then use geometry to relate lattice constant to
atomic radius.
For example, iron foil can be used as a catalyst. The
atomic packing of the exposed plane is important.
a) Draw (100) and (111) crystallographic planes
b) Calculate the planar density for each of these planes.
20
Planar Density of (100) -Iron (Ferrite)
For T < 912C the equilibrium structure of iron is BCC.
2D repeat unit
(100)
a=
4 3
R
3
(from slide 8)
Radius R = 0.1241 nm
atoms
2D repeat unit
Planar Density =
area
2D repeat unit
1
a2
=
1
4 3
R
3
2 = 12.1
atoms
19 atoms
=
1.2
x
10
nm2
m2
Planar Density of (111) Ferrite
2a
h=
3
a
2
2
area = 2 ah = 3 a 2 = 3
atoms
2D repeat unit
=
16 3 2
R
3
1
= 7.0
Planar Density =
area
2D repeat unit
4 3
R
3
16 3
3
R2
atoms =
nm2
0.70 x 1019
atoms
m2
Close-packed planes and structures
• {111} in FCC have metal atoms as close together as possible.
Called close-packed.
• So FCC structure of metals is also sometimes called “cubic close packed.”
• In VMSE:
http://higheredbcs.wiley.com/legacy/college/callister/1118061608/vmse/xtalclose.htm
Watch close-packed {111} planes added to build FCC:
http://departments.kings.edu/chemlab/animation/clospack.html
ABCABCABC , where A, B and C are three possible positions of atoms.
• In L-03B we look at another close-packed structure for metals built of the
same planes, but in a different order, ABABAB.
23