Handout
advertisement

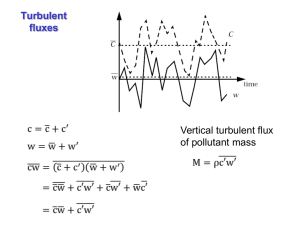
TOX 715: Environmental Toxicology Environmental Transport Transport and Fate of Toxicants in the Environment Transport and fate model Environmental factors that may modify exposure Toxicant Source(s) Exposure-Response Model Toxicant Exposure Toxicant Effects Environmental Compartments Environmental Chemodynamics Fugacity “Tendency of a compound to escape from one environmental compartment into another one” Fugacity Basics Fugacity is to mass diffusion what temperature is to heat transfer. Fugacity is linearly proportional to concentration. Chemicals move from compartments in which they have high fugacities to those of low fugacity. Fugacity Basics When the fugacities of a compound in two adjacent phases are equal, the system is in equilibrium. Fugacity is expressed in units of pressure. Fugacity C1 C0 Partitioning Partitioning C1 K1, 2 C2 Solvent Partitioning Csolvent KP Cwater S solvent KP S water Other Partition Coefficients: Kow Koc, Kb and Kpw Partitioning Theory and the Environment Partitioning can be used to model the distribution of an organic compound in the environment. Examples: ▫ Sorption ▫ Bioconcentration. Bioaccumulation Bioaccumulation ▫ general term used to describe a series of processes by which chemicals found in the environment are accumulated and concentrated in living organisms Bioconcentration Biomagnification Bioconcentration Factors Calculating BCFs log BCF = 0.76 log Kow – 0.23 log BCF = log Kow – 1.32 log BCF = 0.50 log kow – 3.457 Bioavailability Vapor Pressure The pressure that the vapor of a substance exerts on its own liquid or solid state at equilibrium 1 atm = 760 mmHg = 760 torr = 1.013 x 105 Pa. Measuring Vapor Pressures PV = nRT P = nRT/V = 244.4 (n/V) atm ▫ n/V = vapor density in moles/L ▫ R = universal gas constant = 0.082 L atm/°K/mole ▫ T = temperature in the generating column in °K Henry’s Law At equilibrium and at a determined temperature a constant relationship exists between the concentration of a chemical in air and water. Henry’s Law Constant CA P H CW S ' Estimating Volatilization Rates from H’ Adsorption General Characteristics Physico-chemical properties of the sorbent and the adsorptive Area of the sorbent The lower the aqueous solubility of the adsorptive (solute) the higher the binding potential Heat has the potential to reduce adsorption Dissipation C0 ln k1t C t1/ 2 ln 2 0.693 k1 k1 Environmental Transport Advection Refers to the passive movement of a chemical as part of its presence in a medium that is in movement itself. It can happen in the same compartment or between different compartments. Homogeneous Advection J CvW vW flow rate C Concentration Example, consider water in a stream flowing at 1000 m3/h and carrying a chemical at 0.5 μg/m3. The chemical is being advected in water at a rate of 500 μg/h. Heterogeneous Advection Refers to the case where there is a secondary phase present inside the main advective medium. Examples: particulate matter present in advecting river water, particles carried by wind. Diffusion Random movement of chemical molecules due to the presence of a state of disequilibrium. It will transport chemicals from one place to the other one within the same compartment as well as between compartments until equilibrium is reached. Intraphase Diffusion Two types of diffusional intraphase transport: molecular and turbulent diffusion. Molecular diffusion: movement of particles because of a concentration gradient. Turbulent diffusion: happens because of the turbulent mixing of the bulk medium. Intraphase Diffusion Fick’s law: C J DA z J kMAC Interphase Diffusion Diffusion between two phases can be described using the following formula: J kA(C1 C2 K12 ) Transport in Solution Advection Molecular diffusion Turbulent diffusion Dispersion Transport in Solution Advection Molecular diffusion C J DM z 2.7 104 DM 0.71 2 M cm /s Turbulent diffusion Dispersion DM 1 M 20.5 0.5 DM 2 M1 Water-Air Transport Transport between Water-Air Ca J K L CW H' C C0e K L t / z t1/ 2 0.69Z KL Transport through Soil Vadose zone Saturated zone Aquaclude (basal rock) Transport in the Vadose Zone Chemicals are able to migrate through the vadose zone by three main mechanisms: ▫ dissolved in solution ▫ as gases (vapor) ▫ adsorbed to particles Transport through Groundwater Atmospheric Transport Volatilization Advection Deposition Advective Transport Deposition Deposition