Chapter 21
advertisement

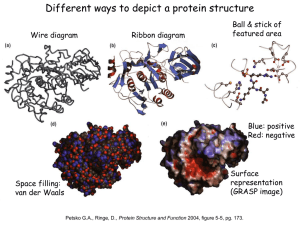
Proteins Chapter 22 Proteins (Greek = “of first importance”) Functions: – Structure - skin, bones, hair, fingernails – Catalysis - biological catalysts are enzymes – Movement - muscle: actin and myosin – Transport - hemoglobin, transport thru membranes Proteins Functions: – Hormones - insulin, oxytocin, HGH, etc. – Protection - antigen-antibody reactions, fibrinogen in clotting – Storage - casein in milk, ovalbumin in eggs, ferritin in liver-stores iron – Regulation - control in expression of genes Proteins • Protein types: – 9000 different proteins in a cell – Individual human being >100,000 different – Fibrous Protein • Insoluble in H2O • Used mainly for structural purposes – Globular Protein • Partly soluble in H2O • Usually not used for structural purposes Proteins are Natural Polymers • Proteins are constructed in the body from many repeating units call amino acids • Just like other polymers the amino acids (monomers) are joined together to make long chains (polymers) – but we call them proteins instead • All of the polymer information applies to proteins – cross linking, rings, polarity etc. Amino Acids • The Building Blocks of proteins – Contains an amino group and an acid group – Nature synthesizes about 20 common AA – All but one (proline) fit this formula: – AA Proline: H R C COOH COOH N proline NH 2 H Amino Acids • Amino Acids (AA) – The twenty common are Called alpha amino acids – One and three letter codes given to 20 common AA – All but glycine (where R=H) exist as a pair of enantiomers • nature usually produces the L amino acid LOOK IN THE BOOK H R C COOH NH 2 Amino Acids • Amino Acids (AA) – Sometimes classified as AA with: • • • • nonpolar R groups polar but neutral R groups acidic R groups basic R groups Zwitterions • An acid -COOH and an amine -NH2 group cannot coexist • The H+ migrates to the -NH2 group • COO- and NH3+ are actually present, called a “Zwitterion” Zwitterions • Zwitterion = compound where both a positive charge and a negative charge exist on the same molecule • AA are ionic compounds • They are internal salts • In solution their form changes depending on the pH AA’s Zwitterions pH = 1-5 pH = 10-14 more basic more acidic excess H+ excess OH- H R C H H COOH NH 3 + R C COO- NH 3 + R C COO- NH 2 AA’s Zwitterions pH = 1-5 pH = 10-14 more basic more acidic excess H+ H R C COOH NH 3 + at pI (isoelectric point) charge = 0 H R C COO- NH 3 + excess OHH R C COO- NH 2 AA’s pI • The pI is the “isoelectric point” • The pI is the pH where NO charge is on the AA: at pI charge = 0 (Not necessarily at a neutral pH) H R C COO- NH 3 + Cysteine • The AA Cysteine exists as a dimer: H 2 HS CH 2 C COOH NH 2 cysteine [O] [H] H HCOO H C CH 2 S S CH 2 C NH 2 cystine COOH NH 2 a disulfide linkage AA’s Peptides • AA are also called peptides • They can be combined to form... H H2N O CH 3 O CH C OH + H2 N CH C OH glycine alanine -H2 O AA’s Peptides • AA are also called peptides • They can be combined to form a dipeptide. H H2N O CH 3 O CH C OH + H2 N CH C OH glycine alanine H H2 N -H2 O O CH C CH 3 O NH CH C OH a peptide bond Peptides • Known as a “dipeptide” H H2 N amine end O CH C CH 3 O NH CH C OH a peptide bond glycylalanine (Gly-Ala), a dipeptide acid end Peptides • Glycylalanine is not the same as Alanylglycine H H2 N O CH C CH 3 O NH CH C OH glycylalanine CH 3 O H2 N CH C H O NH CH C OH alanylglycine Peptides • Synthesis of Alanylglycine CH 3 O H2N CH C OH alanine H + H2N O CH C OH -H2O glycine CH 3 O H2N CH C H O NH CH C OH alanylglycine Peptides • Addition of peptides (head to tail) – Formation of: • dipeptides • tripeptides • tetrapeptides • pentapeptides • polypeptides • PROTEINS AA’s Student Practice • Show the product for the following combination of amino acids Glu – Pro – His Pro – Asn – Leu Val – Ala – Trp http://www.youtube.com/watch?v=va0DNJId _CM Proteins • Proteins usually contain about 30+ AA • AA known as residues – One letter abbreviations • G, A, V, L – Three letter abbreviations • Gly, Ala, Val, Leu • N terminal AA (amine end) on LEFT • C terminal AA (carboxyl end) on RIGHT glycylalanine Gly-Ala G-A AA’s Polypeptides side chains • Polypeptides R R R R R R N CH C N CH C N CH C N CH C N CH C N CH C H O H O H O H O H O H O amino acid residues peptide bonds peptide bonds AA’s Solubility • Polypeptides or Proteins – If there is a charge on a polypeptide, it is more soluble in aqueous solution – If there is NO CHARGE (neutral at pI), it is LEAST SOLUBLE in solution H R C H COOH R C COO- NH 3 + NH 2 charged charged Protein Structure • Primary Structure 1o – Linear sequence of AA • Secondary Structure 2o – Repeating patterns ( helix, pleated sheet) • Tertiary Structure 3o – Overall conformation of protein • Quaternary Structure 4o – Multichained protein structure Protein Structure • Primary Structure 1o – Linear sequence of AA R R R R R R N CH C N CH C N CH C N CH C N CH C N CH C H O H O H O H O H O H O AA 1 AA 2 AA 3 AA 4 AA 5 AA 6 With any 6 AA residues, the number of possible combinations is 6 x 6 x 6 x 6 x 6 x 6 = 46656 AA’s Protein Structure • Primary Structure R R R R R R N CH C N CH C N CH C N CH C N CH C N CH C H O H O H O H O H O H O AA 1 AA 2 AA 3 AA 4 AA 5 AA 6 With any 6 of the 20 common AA residues, the number of possible combinations is 20 x 20 x 20 x 20 x 20 x 20 = 64,000,000 (and this is not nearly large enough to be a protein!) AA’s Protein Structure • Primary Structure – A typical protein could have 60 AA residues. This would have 2060 possible primary sequences. 2060 = 1078 This results in more possibilities for this small protein than there are atoms in the universe! Protein Structure • Primary Structure – Sometimes small changes in the 1o structure do not alter the biological function, sometimes they do. AA’s Changes and Effect of AA change • Cattle and hog insulin is used for humans but is different • Sickle cell anemia – only one change in an amino acid – changes the hemoglobin From yahoo images youtube • http://www.youtube.com/watch?v=bCOJkp L7MVw Protein Structure • Secondary Structure – Repeating patterns within a region – Common patterns helix pleated sheet – Originally proposed by • Linus Pauling • Robert Corey AA’s Protein Structure • Secondary Structure helix – Single protein chain – Shape maintained by intramolecular H bonding between -C=O and H-N– Helical shape • helix is clockwise AA’s YOUTUBE http://www.youtube.com/watch?v=yh9Cr5n2 1EE http://www.youtube.com/watch?v=XKI0le9e2 D8 Protein Structure • Secondary Structure pleated sheet – Several protein chains – Shape maintained by intramolecular H bonding and other attractive forces between chains – Chains run anti-parallel and make U turns at ends AA’s Protein Structure • Secondary Structure • Random Coils – Few proteins have exclusively helix or pleated sheet – Many have non-repeating sections called: Random Coils AA’s Collagen Protein Structure • Secondary Structure • Triple Helix of Collagen – Structural protein of connective tissues • bone, cartilage, tendon • aorta, skin – About 30% of human body’s protein – Triple helix units = tropocollagen AA’s Youtube http://www.youtube.com/watch?v=YmuFI1jtc 8M&feature=PlayList&p=C8887E4E7D367 515&index=0&playnext=1 http://www.youtube.com/watch?v=gXeYf9dL T3s Tertiary Structure – The Three dimensional arrangement of every atom in the molecule – Includes not just the peptide backbone but the side chains as well – These interactions are responsible for the overall folding of the protein – This folding defies its function and it’s reactivity AA’s Tertiary Structure The Tertiary structure is formed by the following interactions: Covalent Bonds Hydrogen Bonding Salt Bridges Hydrophobic Interactions Metal Ion Coordination AA’s Tertiary Structure –Covalent Bonding • The most common covalent bond in forming the tertiary structure is the disufide bond • It is formed from the disulfide Interaction of cysteine H 2 HS CH 2 C COOH NH 2 cysteine [O] [H] H HCOO H C CH 2 S S CH 2 C NH 2 cystine COOH NH 2 Tertiary Structure –Hydrogen Bonding • Anytime you have a hydrogen connected to a F O of N – you can get hydrogen bonding • These interactions can occure on the side chain, backbone or both Tertiary Structure –Salt Bridge • Salt bridges are due to charged portions of the protein. • Opposite charges will attract and Form ionic bonds • Some examples are the NH3+ and COO- areas of the protein Tertiary Structure –hydrophobic interactions • Because the nonopolar groups will turn away from the water and the polar groups toward it, hydrophobic interactions take place. • These interactions are strong enough to help define the overall structure of a protein Tertiary Structure –Metal Ion Coordination • Two side chains with the same charge would normally repel each other • However, if a metal is placed between them, they will coordinate to the meal and be connected together. • These metal coordinations are Important in tertiary structure formation Tertiary Structure Quaternary Structure – Highest level of organization – Determines how subunit fit together – Example Hemoglobin (4 sub chains) • 2 chains 141 AA • 2 chains 146 AA - Example - Collagen Denaturation • Denaturation – Any physical or chemical agent that destroys the conformation of a protein is said to “denature” it – Examples: • • • • Heat (boil an egg) to gelatin Addition of 6M Urea (breaks H bonds) Detergents (surface-active agents) Reducing agents (break -S-S- bonds) Denaturation • Denaturation – Examples: • Acids/Bases/Salts (affect salt bridges) • Heavy metal ions (Hg2+, Pb2+) – Some denaturation is reversible • Urea (6M) then add to H2O – Some is irreversible • Hard boiling an egg Denaturation • Denaturation