2 - EngineeringDuniya.com
Matter waves
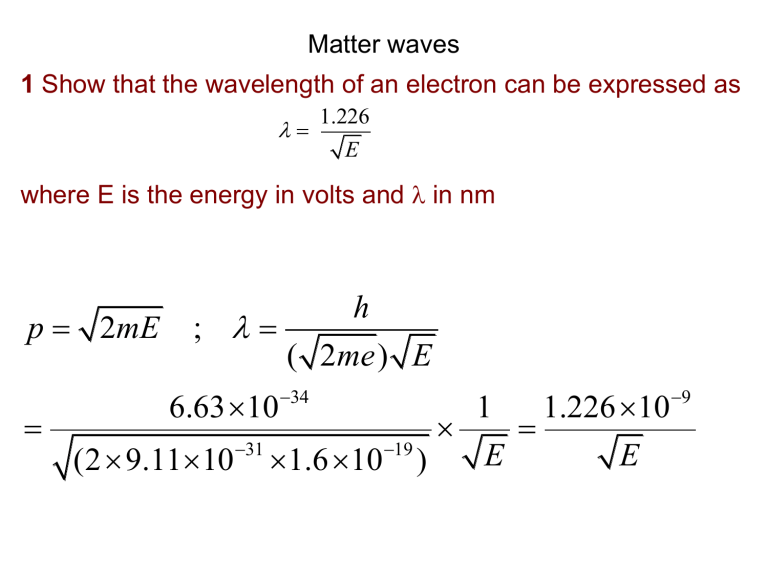
1 Show that the wavelength of an electron can be expressed as
1.226
E where E is the energy in volts and
in nm p
2 mE ;
h
( 2 me ) E
34
(2 9.11 10
31
19
)
1
E
1.226 10
E
9
Matter waves
2 Show that the wavelength of an electron of energy E can be expressed as
1.50
V
V where V is the accelerating potential in volts and
in nm
p
2 meV ;
h
( 2 )
34
31
19
(2 9.11 10 1.6 10 )
1
V
1.50 10
V
9
Matter waves
3 Calculate the de Broglie wavelength of
( i) An electron with energy (a) 10 eV (b) 15 keV (c) 1 MeV
(i) (a)
1.226
E
(E in eV)
1.226
0.387
nm
387 pm
10
(b)
1.226
3
0.0100
nm
10 pm
Matter waves
3 Calculate the de Broglie wavelength of
(c )An electron with energy 1 MeV
(c )
E
2 pc
2 m c
2 2
( ) ( )
0 u sin g relativistic relation p
1 c
E
2 m c
2 2
( )
0
hc p
E
2 m c
2 2
( )
0
6.63 10
34
3 10
8
6 2 3 2
1.45
pm
19 2
Matter waves
3 Calculate the de Broglie wavelength of
(ii) An electron moving with a speed of 105 m/s
(iii) A proton with an energy of 15 eV
(ii)
=(h/p)=(h/mv)=( 6.63
10 -34 )/(9.11
10 -31
105)
= 6.93
10 -6 m
(iii)
=h /p = 6.63
10 -34 /
[2mE]
= 6.63
10 -34 /
[2
( 1.67
10 -27
)(15)
1.6
10 -19 )]
= 7.4
10 -12 m =7.4pm
Matter waves
3 Calculate the de Broglie wavelength of
(iv)
A neutron with an energy of 10 keV
=h /p = 6.63
10 -34 /
[2mE]
= 6.63
10 -34 /
[2
( 1.67
10 -27
)(10
10 3 )
1.6
10 -19 )]= 2.87
10 -13 m
(v) A neutron moving with a speed of 104 m/s
=h /p = h /mv = 6.63
10 -34 / ( 1.67
10 -27
)
104 = 3.8nm
(vi) A ball of 45 g moving with a speed of 22 m/s
=h /p = 6.63
10 -34 / 45
10 -3
22 =6.7 x 10
–34 m
(vii) A bullet of 5 g moving with a speed of 200 m/s
=h /p = 6.63
10 -34 / 5
10 -3
200 =6.6 x 10
–34 m
Matter waves
4 Compute the accelerating potential required to produce an electron beam of de Broglie wavelength of 10 pm
1.50
V
V
V = 1.5/
2 = 15 kV
Davisson and Germer experiment
5 A crystal is cut such that the rows of atoms in its surface are separated by a distance of 0.352nm.
A beam of electrons is accelerated through a potential difference of 175 V and is incident normally on the surface.
At what angles relative to the incident beam would the diffracted beams be observed?
Solution
( nm )
1.5
V
1.5
V
0.0926
nm
V 175 d sin
=n
d=lattice spacing,
= wavelength of matter wave;
= angle between the incident & the scattered beam sin
= (
/d) n ; (0.0926nm/0.352nm)n = 0.263n
=sin -1 (0.263n) = 15.3
, 31.8
,52.4
Matter waves
6 Show that the de Broglie wavelength of a particle of rest mass m
0 energy K is given by and kinetic
E
2
( pc )
2
( m c
0
) ;
h
hc p
E
2 m c
2 2
( )
0
h c
K K
2 m c
2
) p
1 c
E
2
( m c
0
) hc
( K
m c
0
2 2 m c
2 2
) ( )
0
hc
( K
m c
0
2 m c
0
2
)( K
m c
0
2 m c
0
2
) hc
K K
m c
2
( 2 )
0
Wave packet
7 Certain ocean waves travel with a phase velocity g
2
Where, g is the acceleration due to gravity. Find the group velocity of a wave packet of these waves in terms of the phase velocity.
v p
v g
d g
2
dk
g k dk g
) k d ( gk )
dk
1
2( gk )
g
1
2
g
k
1
2 v p
Wave packet
8 An electron has a de Broglie wavelength of 2.00nm.
Find its kinetic energy, phase velocity and the group velocity of the de Broglie waves
.
KE=p 2 /2m =(h/
) 2 /2m
=( 6.63
10 -34 /(2
10 -12 ) 2 /(2
9.11
10 -31 )
= 6.038
10 -20 J=0.377eV
V g
=
(2E/m)
=
(2
6.038
10 -20 )/(9.11
10 -31 )
= 3.64
10 5 m/s v p
= c 2 /v g
=2.5
10 11 m/s
Wave packet
8 An electron has a de Broglie wavelength of 2.00pm. Find its kinetic energy, phase velocity and the group velocity of the de Broglie waves
.
(use relativistic relation) p = h /
=3.315
10 -22 m kg/s; pc = 9.945
10 -14 J
E 2 = ( pc) 2 + (m
0 c
E =
[( pc) 2 + (m
0
2 c
)
2
2
)
; =9.89
2
10
]= 1.287
-27
10
+6.684
-13 J
10 -27
KE= Total energy (E)-Rest energy (m
E = m c 2 = (m
0 c 2) /
[1- v 2 / c 2 ]
0 c 2 )= 4.698
10 -14 J
[1- v 2 / c 2 ] = (m
0 c 2 ) 2 / E 2 =
v 2 = c 2 [1- (m
0
V = c
[1- (m
0 c c
2
2 )
) 2
2
/ E
/ E
2
2 ]
]
= 3
10 8
[1- {(511
10 3
1.6
10 -19 J)/ 1.287
10 -13 }
=2.3
10 8 m /s
Heisenberg uncertainty principle
3 In a gamma decay process the life time of decaying nuclei is 2 x 10
– 20 s.
What is the uncertainty in the energy of the gamma rays emitted ?
(
E)(
t) ≥ (h/4
) ;
E ≥ h/(4
)(
t)
E ≥ 6.63
10 -34 /[4
2
10 -20 ] = 2.6 x 10 –15 J = 16 keV
4 An excited atom radiates a quantum of light of certain wavelength. Taking 10 ns to be the uncertainty in the time of radiation calculate the uncertainty in the frequency of the light emitted. Answer: 7.96 MHz
(
E)(
t) ≥ (h/4
) ;
E ≥ h/(4
)(
t) ; h
≥ 6.63
10 -34 /[4
10
10 -10
6.63
10 -34 ] = 7.96MHz
Heisenberg uncertainty principle
7 If the uncertainty in the location of a particle is equal to its de
Broglie wavelength, what is the uncertainty in its velocity?
Solution:
(
p x
)(
x) ≥ (h/4
) ;
v x
v x
≥ h /[4 m
] ;
v x
≥ h/(4
)(m )(
x)
= (h/
) / [4
m ]= (p/m)/ 4
= v/ 4
8 A spectral line of wavelength 4000 Å has a width of 8
10 -5 Å. Calculate the minimum time spent by the electron in the upper energy state between the excitation and the de-excitation processes.
(
E)(
t) ≥ (h/4
) ; E = hc/
;
E = -(hc/
2 )
t ≥ h
2 /[(4
)(hc)(
)]
≥
2 /[(4
)(c)(
)]
t ≥(4000
10 -10 )2 /[4
3
10 8
( 8
10 -5
10 -10 )] = 5.3x 10
–9 s
Heisenberg uncertainty principle
9 A typical atomic nucleus is about 5.00
10
–15 m in radius.
Use the uncertainty principle to place a lower limit on the energy an electron must have if it is to be a part of a nucleus.
(
p x
)(
x) ≥ (h/4
) ;
p x
p x
≥ h/(4
≥ h /[4
5.00
2
10
–15 ];
p x
)(
x)
= 5.275
10
–21 kg .m/s p min
= 5.275
E min
2
E min
= (pc) 2
10
= 3.166
–21
+ (m
10 kg .m/s
0
–12 c) 2 = 1.0023
J= 10MeV
10
–23
Infinite potential well
5 An electron is bound in a one-dimensional potential well of width,
L = 100 pm (a typical atomic diameter), but of infinite wall height.
(a) Find its energy values in the ground state and also in the first two excited states.
Solution
(a) E=
E
0
2 2 n h
8 mL
2
2
8 h mL
2
=
2 n E
0
8
9.11
[6.6 3
10
34 2
]
10
34
(100
10
12
)
2
E
1
6.03
4
E
0
10
18
J
37.7
eV
150.78
eV ;
E
2
9 E
0
339.3
eV
Infinite potential well
(b) How much energy must be supplied to excite the electron from the ground state to the first excited state?
E
E
0 h
2
n
1
2 n
0
2
8 mL
2
[4
1]
37.7
113.1
eV
(c) Estimate the probability of finding the electron in the ground state over a stretch of 10 pm each at distances x = L / 2, L /3,
L/ 4 and L from one end of the wall.
Probability density = P =
2 = (2 / L)[sin 2 [(n
/L)x]
For ground sate n=1
At x=L/2
P = (2 / L)[sin 2 [(
/L)(L/2)] = 2 / L
P(dx) = (2/100)
10 = 0.20
Infinite potential well
(c) Estimate the probability of finding the electron in the ground state over a stretch of 10 pm each at distances x = L / 2, L /3, L/ 4 and L from one end of the wall.
At x=L/3
P= (2 / L)[sin 2 [(
/L)(L/3)] =( 2 / L)(3/4)=1/L
P(dx) =(3/ 2
100)(10) = 0.15
At x=L/4
P= (2 / L)[sin 2 [(
/L)(L/4)] =( 2 / L)(1/2)=1/L
P(dx) =(1/100)(10) =0.10
At x=L
P= (2 / L)[sin 2 [(
/L)(L)] = (2/L)
0
P(dx)= 0
Infinite potential well
(d) In the first excited state what is the probability of finding the electron between x
1
= 0 and x
2
= 25 pm?
(
1, 2
)
2
L x
2 x
1
2
{sin ( n
L x dx
1
L x
2 x
1
{1
cos(
2 n
L
1
L
x
2 x
1
L
2 n
sin(
2 n
L
) x x
2
x
1
Infinite potential well
For the first excited state n=2; Limits X
1
=0 and x
2
=25pm =L/4
Substituting the value of n and the limits
P x x
1, 2
)
1
L
L
0
4
4
L
4
sin( )
L x
0
L
4
1
L
L
4
0
L
4
1 1
L
0.25
L
sin(
4
L
)
L
4
sin(
4
L
)
L
4
L
4
sin
sin 0
1
4
1
P =0.25
Infinite potential well
(e)
What is the difference in the values of momentum of the electron in the ground state and 2
nd
excited state?
( P
2
P
0
)
2 mE
2
2 mE
0
2 mE n
0
2
2 mE
0
n
2
1
2 mE
0
2
31
6.01 10
18
3
1
34
.
/
Infinite potential well
(e)
What is the difference in the values of velocity of the electron in the ground state and 2
nd
excited state?
(
2
v
0
)
2 E
2 m
2 E n
0
2 n
2
m
2 E
2 m
2 E n
0
2 m
2 E
0 m
18
31 m
6 m s
Infinite potential well
(e)
What is the difference in the values of (iii) de
Broglie wavelength of the electron in the ground state and 2
nd
excited state?
2
0
)
h
2 mE
0
1 n
2
1
h
2 mE
2 h
2 mE
0 h
2 mE
0
1 n
2
2 h mE n
0
2
h
2 mE
0
10
133 pm
Infinite potential well
(f) What are the values the uncertainty in the position of the electron and its momentum?
(
p x
)(
x) ≥ (h/4
) ;
p x
≥ h/(4
)(
x)
p
p x x
≥ h /[4
1 00
10
–12 ] ;
= 5.28
10
–25 kg .m/s
Infinite potential well
(g) What are the values of the probability density at the mid point of the region, in the ground state and in the first excited state?
Probability density P(x) =
2
2 sin 2 n
L L
At x
L / 2 and n
x
2 ( first excited state )
2
L sin
2
2
L
L
2
0
At x
L / 2 and n
2
2 sin
2 n
L L
At x
L / 2 and n
1 ( ground state ) x
1
2
L sin 2
L
L
2
2
L
2 10 10
Infinite potential well
6 Compare the ground state momenta, the ground state kinetic energy and the uncertainties in the velocities of an electron and a proton (mass =
1840 x electron mass) confined in a one dimensional “box” of length
1 .
0 nm
.
Momenta:
P e
P p
h
e h
p
1
e
e
1 nm
Kinetic energy:
K e
K p
p e
2
2 m e p p
2
2 m p
m p m e
1840
Uncertainty:
v e
v p
2 m e
x e
2 m p
x p
m p m e
1840
x e
x p p e
p p
Infinite potential well
9
An electron is trapped in an infinitely deep one-dimensional potential well of width 0.251 nm. Initially electron occupies the n ═ 4 state. Suppose the electron jumps to the ground state, with the emission of a photon.
(a) what is the energy of the photon?
Solution h 2
E
E n
0
2
; E
0
8 mL
2
[6.6 3
10
34 2
]
8
9.11
10
34
(0.251
10
9 2
)
9.57
10
19 J
E
(3
0 )
h
2
8 mL
2
9.57
10
19
n
3
2 n
0
2
16
1
90 eV
Infinite potential well
9
An electron is trapped in an infinitely deep one-dimensional potential well of width 0.251 nm. Initially electron occupies the n ═ 4 state. Suppose the electron jumps to the ground state, with the emission of a photon.
(b) What is the difference in the velocity of the electron in the two states ?
Solution
(
v
2 0
)
2 E m
2
2 E m
2
2 E n m
0
2
2 E m
0
2 E n
0
2 m
n
2
31 m
19 6 m s
Infinite potential well
9
An electron is trapped in an infinitely deep one-dimensional potential well of width 0.251 nm. Initially electron occupies the n ═ 4 state. Suppose the electron jumps to the ground state, with the emission of a photon.
(c )What is the difference in the wavelength of the electron in the two states?
Solution
( iii )(
2
0
)
h
2 mE
2
h
2 mE
0
h
h
2 mE n
0
2 2 mE
0
2 h mE
0
1 n
2
1
h
2 m
9.57
10
19
3.77
10
10 m
377
1
16 pm
1
Infinite potential well
9
An electron is trapped in an infinitely deep one-dimensional potential well of width 0.251 nm. Initially electron occupies the n ═ 4 state. Suppose the electron jumps to the ground state, with the emission of a photon.
(d) How much is the uncertainty in the velocity of the electron in the potential well.
Solution
(
Uncertainty in velocity=
v x
x)(
p x
) ≥ (h/4
) ;
v x
≥ h/(4
)(m
x)
v x
≥ 6.63
10 -34 /[4
9.11
10 -31
0.251
10 -9 ]
≥ 230734
Potential barrier
8 A 30-eV electron is incident on a square barrier of height 40 eV. What is the probability that the electron will tunnel through the barrier if its width is (a)
1.0 nm? (b) 0.10 nm?
Solution:
Transmission coefficient T ≈ e –2k
2
L k
2
2 (
E )
2
2 9.11 10
31
19
4
[6.63 10
]
2
For L
1 nm
T
e
32.4
8.5 10
15
For L
0.1
nm
T
e
3.24
0.039
10
Potential barrier
18 Electrons with energies of 0.400eV are incident on a barrier 3.00eV
high and 0.100nm wide. Find the approximate probability for these electrons to penetrate the barrier
.
Transmission coefficient T ≈ e –2k
2
L k
2
2 (
E )
2
2 9.11 10
31
19
34 2
[6.63 10 ]
For L
0.1
nm
T
e
1.65
0.192
2
9









