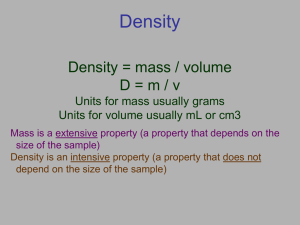
D=2 g/cm 3 - MPMS Pumas` Science
advertisement

Science Friday, October 18th (week 8) Day: 11hr 10min Rise: 7:24 Moon: full moon, 100% illuminated Objectives: I will review matter. Set:6:35 Warm Up! Question: Use your vocabulary sheet to create a concept map/web for this unit. Important Information Due Today: Nothing! Homework Tonight: Studying is always a good idea. Upcoming Events: What the Matter test Wednesday! End of quarter Friday! Grades close Friday. Just what you’ve all been waiting for… The Posttest!!!!! 1) A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is its density? D=2.7g/mL 2) Mercury metal is poured into a graduated cylinder that holds exactly 22.5 mL. The mercury used to fill the cylinder weighs 306.0 g. From this information, calculate the density of mercury. D=13.6 g/mL 3) What is the weight of the ethyl alcohol that exactly fills a 200.0 mL container? The density of ethyl alcohol is 0.789 g/mL. M=157.8 g 4) A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper? D=8.92 g/cm3 5) A flask that weighs 345.8 g is filled with 225 mL of carbon tetrachloride. The weight of the flask and carbon tetrachloride is found to be 703.55 g. From this information, calculate the density of carbon tetrachloride. D= 1.59 g/mL 6) Calculate the density of sulfuric acid if 35.4 mL of the acid weighs 65.14 g. D=1.84 g/mL 7) Find the mass of 250.0 mL of benzene. The density of benzene is 0.8765 g/mL. M= 219.13 g 8) A block of lead has dimensions of 4.50 cm by 5.20 cm by 6.00 cm. The block weighs 1587 g. From this information, calculate the density of lead. D=11.3 g/cm3 9) 28.5 g of iron shot is added to a graduated cylinder containing 45.50 mL of water. The water level rises to the 49.10 mL mark, from this information, calculate the density of iron. D=7.92 g/mL 10) What volume of silver metal will weigh exactly 2500.0 g. The density of silver is 10.5 g/cm3. V=238.1 cm3 12) You are given the following information: mass = 48 g; volume=24 cm3. What is the density of this substance? D=2 g/cm3 13. If you have a rectangular gold brick that is 2 cm by 3 cm by 4 cm and has a mass of 48 g, what is its density? D=2 g/cm3 14. Bob, who weighs 150 pounds, found a rock. What is the density of a rock if its mass is 36 g and its volume is 12 cm3? D=3 g/cm3 15. If a block of wood has a density of 0.6 g/cm3 and a mass of 120 g, what is its volume? V=200 cm3 16. What is the mass of an object that has a volume of 34 cm3 and a density of 6 g/cm3? M=204 g 17. 18. 19. 20. 21. 22. 23. D A K L N M J 24. 25. 26. 27. 28. 29. 30. F I C E H B G 1. Kool-Aid dissolved into water is always a delicious treat. 2. The water passing over the earth’s surface for millions of years created the Grand Canyon. 3. Fish use the oxygen that is dissolved in the water. 4. Sodas come from the plant full of carbon dioxide. That’s why after we open them; if we shake them up we still see bubbles. 5. I like to drink homemade chocolate milk. 6. My mom always tells me to gargle with salt water when I have a sore throat. 7. Acid rain is by pollutants mixing with rain. 8. I never put powdered lemonade in my water bottle; it tastes like detergent. 9. Sometimes we can have minerals and metals that may be dissolved in water. This may cause tap water to taste as if pennies were soaked in it. 10. She hates the taste of barbeque sauce since it has ketchup and Worcestershire sauce in it. Gas Melting Solid Freezing Liquid QN vs QL 1. It was so cold I had to wear gloves! QL 2. She is 5 feet 9 inches tall. QN 3. The outlet has 2 plugs. QN 4. There are 23 Smarties in a roll. QN 5. His hair is pink. QL 6. It takes me 23 minutes to get to school. QN 7. I spent tons of money on groceries yesterday. 8. The dog weighs 18 pounds! QN 9. The class average was low. QL 10. She scored a 9 out of 10. QN QL 1.What is the connection between a student’s GPA and the amount of time they spend watching TV each week? 2.The amount of time spent watching TV 3.The student’s GPA 4.Same amount of sleep each night Problem Independent Variable Dependent Variable 1 2 3 Constant or Controlled Variable 4 5. The amount of rain 6. Do grapes taste sweeter if there is less rain during the growing season? 7. The same type of grape 8. The sweetness of the grapes Problem Independent Variable Dependent Variable Constant or Controlled Variable 6 5 8 7 9. The amount of light that each plant receives 10. The same type of tomato plant used 11. Does the amount of sunlight a tomato plant receives affect the number of tomatoes produced? 12. The number of tomatoes produced. Problem Independent Variable Dependent Variable Constant or Controlled Variable 11 9 12 10 Definition Name of Step The reason you are doing this Purpose experiment. Educated guess/prediction as to Hypothesis what the results will be. When you gather information and Research materials needed for this lab. Steps used to design and perform Procedure an experiment. A summary or answer to the Conclusion problem based on the data. Scientists should never assume that Repeat the the first result will always occur. work Data that was collected and analyzed Results during the experiment. Order 1 3 2 4 6 7 5