q = mcΔT
advertisement
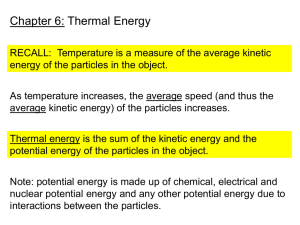
Thermal Energy Thermal Energy • Temperature & Heat • Temperature is related to the average kinetic energy of the particles in a substance. • SI unit for temp. is the Kelvin • K = °C + 273 (10°C = 283K) • °C = K – 273 (10K = -263°C) • Thermal Energy – the total of all the kinetic and potential energy of all the particles in a substance. • Thermal energy relationships • As temperature increases, so does thermal energy (because the kinetic energy of the particles increased). • Even if the temperature doesn’t change, the thermal energy in a more massive substance is higher (because it is a total measure of energy). Heat Cup gets cooler while hand gets warmer •The flow of thermal energy from one object to another. • Heat always flows from warmer to cooler objects. Ice gets warmer while hand gets cooler Types of Heat Transfer 3 WAYS THAT HEAT CAN TRAVEL SONG: • https://www.youtube.com/watch?v=7Y3mf AGVn1c Types of Heat Transfer Conduction Conduction is the transfer of heat by direct contact. *occurs best in solids Types of Heat Transfer Convection • Convection is the transfer of heat through fluids by currents created due to density differences in parts of the fluid. *occurs in liquids and gases only Hot water rises, cools and falls. Heated air rises, cools and then falls. Cool air falls. Types of Heat Transfer Radiation • Radiation is the transfer of heat energy through space as waves of electromagnetic radiation. *the only type that can occur through empty space (example: from the sun) Specific Heat • Some things heat up or cool down faster than others. Land heats up and cools down faster than water. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). C water = 4184 J / kg C C sand = 664 J / kg C This is why land heats up quickly during the day and cools quickly at night and why water takes longer. Specific Heat • Which take longer to heat to 100°C? 50g Al 50g Cu C Water Aluminum Copper Silver Gold C (cal/g°C) (J/g°C) 1.00 0.22 0.093 0.057 0.031 4.18 0.90 0.39 0.24 0.13 Aluminum has a higher specific heat, so it will take longer to heat up. It will ALSO take longer to cool down. Why does water have such a high specific heat? water metal Water molecules form strong bonds with each other; therefore it takes more heat energy to break them. Metals have weak bonds and do not need as much energy to break them. How to calculate changes in thermal energy q = mCpT q = change in thermal energy (heat) m = mass of substance T = change in temperature (Tf – Ti) Cp = specific heat of substance -q means heat loss +q = heat gain Heat Transfer A 32g silver spoon cools from 60°C to 20°C. How much heat is lost by the spoon? GIVEN m = 32g Ti = 60°C Tf = 20°C q = ?? C = 0.235 J/g°C WORK q = mCΔT m = 32g ΔT = Tf - Ti ΔT = 20°C – 60°C = -40°C q = (32g)(0.235J/g°C)(-40°C) q = -300.8 J Heat Transfer How much heat is required to warm 230 g of water from 12°C to 90°C? GIVEN m = 230g Ti = 12°C Tf = 90°C Q = ?? C = 4.184 J/g°C WORK q = mCΔT m = 230g ΔT = Tf - Ti ΔT = 90°C – 12°C = 78°C q = (230g)(4.184J/g°C)(78°C) q = 78,061 J A piece of iron at a temperature of 145°C cools off to 45°C. If the iron has a mass of 10 g and a specific heat of 0.449 J/g°C, how much heat is given up? GIVEN m = 10g Ti = 145°C Tf = 45°C q = ?? C = 0.449 J/g°C WORK q = mCΔT m = 10g ΔT = Tf - Ti ΔT = 45°C – 145°C = -100°C q = (10g)(0.449J/g°C)(-100°C) q = -449 J A calorimeter is used to help measure the specific heat of a substance. Heat gained = Heat lost First, mass and Knowing its q value, temperature of Thengives heated its mass, and its This the water are measured T is measured sample put T, its Cp can be heat lostisby the for water to help inside and heat calculated substance get its heat gain flows into water Let’s Practice! A 55.1 g piece of metal is heated to a temp of 45.1°C, and placed into a cup containing 359g of water at 20.0°C. The final temp of the water and metal is 22.3°C. • How much heat energy did the water absorb? q = mcΔT q = (359g)(4.18J/g°C)(22.3°C – 20.0°C) = 3.45 x 103J • How much heat energy did the metal release to the water? q lost = q gained q lost by the metal = - 3.45 x 103J The q is negative because heat was lost. • What is the specific heat of the metal? 3.45 x 103J = (55.1g)(C)(22.3°C – 45.1°C) 2.75 J/g°C = C Heat of Fusion They Quantity of heat absorbed when a specific quantity of the solid is converted to liquid at its melting point is called its heat of fusion. Heat of Vaporization • The quantity of heat absorbed when a specific quantity of the liquid is converted to gas at the boiling point is called the heat of vaporization. Phase Diagram Water boils Ice metls Fusion Vaporization