Chpt 6: Thermal Energy
advertisement
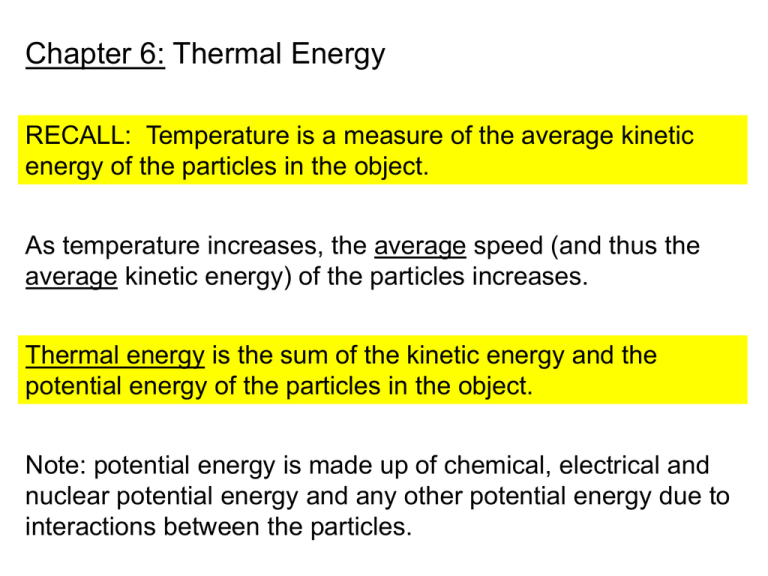
Chapter 6: Thermal Energy RECALL: Temperature is a measure of the average kinetic energy of the particles in the object. As temperature increases, the average speed (and thus the average kinetic energy) of the particles increases. Thermal energy is the sum of the kinetic energy and the potential energy of the particles in the object. Note: potential energy is made up of chemical, electrical and nuclear potential energy and any other potential energy due to interactions between the particles. Thermal energy, Temperature and Mass T = 25 °C T = 25 °C More thermal E! 50 g 100 g Q: Is the thermal energy of both beakers the same? A: No. While both beakers have the same average kinetic energy (same T), there are more particles in the 100 g sample of water so it must have more thermal energy. Heat: thermal energy that flows FROM a region of higher temperature TO a region of lower temperature. As the temperature difference increases, the amount of heat transferred increases. HEAT Hot Very Hot DT1 Cool DT2 > DT1 MORE HEAT DT2 DT = difference in temperature Cold Specific Heat: The amount of heat needed to raise 1 kg of a substance by 1°C Substance Specific Heat [J/(kg°C)] Substance Specific Heat [J/(kg°C)] Water 4184 Iron 450 Wood 1760 Copper 385 Carbon 710 Silver 235 Glass 664 Lead 129 Water is often used as a coolant because of its high specific heat. Substance Specific Heat [J/(kg°C)] Substance Specific Heat [J/(kg°C)] Water 4184 Iron 450 Wood 1760 Copper 385 Carbon 710 Silver 235 Glass 664 Lead 129 Q: What will heat up faster, water or iron? Iron: it has a lower specific heat Q: What will stay hot longer, copper or silver? Copper: it has a higher specific heat Calculating changes in thermal energy Q = mCDT Q = heat energy, units are Joules (J) m = mass, units are kg C = specific heat, units are J/(kg°C) DT = change in temperature (Tf - Ti) EX: A room containing 72 kg of air with a specific heat of 1010 J/kg°C is heated from 20°C to 25°C. How much thermal energy was used? m = 72 kg C = 1010 J/kg°C DT = 25°C - 20°C = 5°C Q = mCDT = (72 kg)(1010 J/kg°C)(5°C) = 363,600 J Calorimetry: A technique used to measure the amount of heat transferred between substances. Thermometer Air Water Metal pieces Stirrer 0.025 kg of metal is heated to 100°C and then put into 0.1 kg of water that is at 20°C. The excess heat in the metal is transferred to the water warming it up to 23°C. 1. How much heat was transferred? QH2O = mCDT QH2O =(0.1 kg)(4184 J/kg°C)(3°C) QH2O = 1255 J 2. What is the specific heat of the 0.025 kg of metal? • If 1255 J of heat was gained by the water, that means that 1255 J of heat was lost by the metal (Conservation of Energy). What is DT? Qmetal = 1255 J • Metal started out at 100°C and ended up at the same temperature as the water, 23°C. DT = 100°C - 23°C = 77°C • Substitute the values into Qmetal = mCDT and solve for C. Qmetal = mCDT 1255 J = (0.025 kg) C (77°C) 1255 J = (1.93) C C = 650 J/kg°C Thermodynamics: describe the inter-relationship between heat, thermal energy and work. First law of thermodynamics: The increase in thermal energy (DU) of a system is the sum of the work done on the system and the heat transferred to the system DU = Q + W In closed systems, heat cannot enter or leave the system. DU = 0 In open systems, heat can be exchanged with the environment DU 0 Second law of thermodynamics: It is impossible for heat to flow from a cool object to a warm object unless work is done on the system. HEAT HOT COLD NO WORK = NO HEAT TRANSFER Section 2: Transferring Thermal Energy Heat Conduction: heat is transferred through a material (glass, wood, metal etc.) Rate of heat transfer depends upon the material. Metals: FAST, good conductors Gases: SLOW, good insulators Convection: transfer of thermal energy in liquids and gases by movement of warmer and cooler regions from place to place. • The warm water is less dense than the cooler water around it so it rises. • The warm water cools (through conduction) as it rises. • The cooled water sinks back to the bottom. Radiation: transfer of energy by electromagnetic radiation. No matter is needed for transfer to occur. • When solar radiation strikes a light colored object, most of the radiation is reflected. The small amount that is absorbed causes a small temperature increase. • When solar radiation strikes a dark colored object, most of the radiation is absorbed. This causes a larger temperature increase. • Because the molecules are far apart, gases do not absorb much solar radiation. Heating systems Forced-Air Steam/hot water Radiators Electric Heating Passive Solar Heating Limitations for both types: • Doesn’t work at night • Doesn’t work well on cloudy days Active Solar Heating Heat Engine: a device that converts heat into work Example: An internal combustion engine • Chemical energy of gasoline is converted into heat, which causes gases to expand and push (a force) against the piston heads. When the pistons move under the force of the expanding gases, work is being done. • Only about 25% of the heat generated by the engine is converted into work. The rest is transferred out to the surroundings. Q: Is an internal combustion engine a closed or open system? A: Heat is transferred to the surroundings, so it is an OPEN system. Heat movers: do work in order to move heat from a COLD area to a WARMER area. Example: A refrigerator • Electrical energy is used to compress the coolant into a liquid. B • The liquid coolant absorbs some of the the thermal energy from the inside of the refrigerator A so it can expand into a gas. This causes the inside of the refrigerator to cool. Air conditioners and heat pumps are also heat movers.











