Lecture 15
advertisement

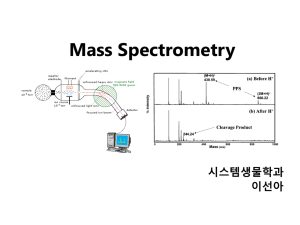
MS Intro MS Intro MS requires gas-phase ions, why? MS uses magnetic and electric fields to control the path of a compound based on mass to charge ratio (m/z) O O O O OH OH O O OH pH 3 pH 7 m/z = 137.11192 m/z = 68.0553 MW = 138.1278 Remember you calculate mass using the most abundant isotope MS Intro MS requires gas-phase ions, why? MS uses magnetic and electric fields to control the path of a compound based on mass to charge ratio (m/z) O O O OH NH2 MW = 137.1438 O O NH2 pH 7 m/z = 136.1351 O OH NH3 pH 1 m/z = 138.1525 NH3 pH 4 m/z = N/A MS Intro MS requires gas-phase ions MS uses magnetic and electric fields to control the path of a compound based on mass to charge ratio (m/z), how? Variety of ways: 1. Control which ions hit the detector - Quadrupole, magnetic sector, ion trap, accelerator MS 2. Determine how fast an ion hits a detector - Time of flight 3. Measure frequency of orbital motion of trapped ions - Fourier transform ion cyclotron resonance (FT-ICR) MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 Resolving power is the ability to separate two neighboring peaks. This is a measure of how ‘sharpness’ of a mass peak. Example… MS Intro Resolving power (RP) m (average mass) RP = m (mass difference) m m MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 Resolving power is the ability to separate two neighboring peaks. This is a measure of how ‘sharpness’ of a mass peak. Example… MS Intro Resolving power (RP) m RP = m Need to determine the accurate mass of both compounds using the most abundant isotopes of each element C = 12.00000 amu H = 1.007825 amu O = 15.994915 amu P = 30.973762 amu S = 31.972072 amu How much resolving power do we need to distinguish between the following compounds: O O P OH 3C + 8H + P+ 3O = 123.021107 amu O S (123.021107 + 123.011592)/2 RP = (123.021107 - 123.011592) RP = 12900 O O 3C + 7H + S + 3O = 123.011592 amu MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 Resolving power is the ability to separate two neighboring peaks. This is a measure of how ‘sharpness’ of a mass peak. Example… MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 Mass accuracy Comparison of the measured mass to the accurate mass Example… MS Intro Mass Accuracy (ppm) 5 ppm = ppm value? 1,000,000 5 1,000,000 = Mass accuracy to 4 decimal places Lower the ppm value the more accurate the measurement = 6 x 10-4 Require < 10 ppm accuracy for ‘accurate mass’ measurement measured mass - actual mass actual mass Example, you have a mass for an unknown compound which you expect is one of the compounds below, which ones is it most likely? O O O Measured mass: 123.0204 P OH MW = 123.021107 amu S O O MW = 123.011592 amu MS Intro Example, you have a mass for an unknown compound which you expect is one of the compounds below, which ones is it most likely? What is the accuracy (ppm) of this measurement as compared to the 2 actual masses ppm value? 1,000,000 measured mass - actual mass = actual mass O O (123.0204 - 123.021107) P OH ppm = x 1,000,000 = 5.7 123.021107 MW = 123.021107 amu O S (123.0204 - 123.011592) O O MW = 123.011592 amu ppm = 123.011592 x 1,000,000 = 72 MS Intro Characteristic Quadrupole Ion trap Magnetic Sector Time of Flight FT-ICR Mass Range (Da) < 4000 < 4000 15,000 Unlimited >104 Resolving power 4000 103-104 102-105 15,000 >106 Mass Accuracy (ppm) 100 50-100 1-5 5-50 1-5 Mass accuracy Measured error in m/q divided by the accurate mass Example… Quadrupole MS Quadrupoles Ion focusing +- +- +- + - RF only - All ions pass Quadrupoles Ion focusing - RF only - All ions pass Quadrupole MS -+ -+ How do the quads work? - A DC potential is applied to the poles, making 2 diagonal poles negative and 2 positive - The RF potential is superimposed over the DC potential -+ -+ Quadrupole MS RF pulse +- ++ + How do they select for an m/z? - Select for m/z 500 (positive) - positive ion will be attracted to a negative pole - during the RF pulse the charges of the poles reverse, and the ion will be repelled from this same pole + - ion will be attracted to a pole that is negative +- + - this process continues as the ion moves through the poles (into the slide) Quadrupole MS + ++ + + +- + How do they select for an m/z? - Select for m/z 500 (positive) - positively charged poles focus ions into centre plane - negatively charged poles attract ions out of the plane Quadrupole MS RF pulse +- + + + + + + + + How do they select for an m/z? - Select for m/z 500 (positive) - positively charged poles focus ions into centre plane - negatively charged poles attract ions out of the plane - during the RF pulse these charges reverse and attractive / repulsive forces reverse and the ions are focused in the other plane Quadrupole MS + +- + +- + How do they select for an m/z? - Select for m/z 500 (positive) - positively charged poles focus ions into centre plane - negatively charged poles attract ions out of the plane - during the RF pulse these charges reverse and attractive / repulsive forces reverse and the ions are focused in the other plane - results in a complicated path with the ion moving towards and away from the poles as the potential changes, but constantly being refocused into the centre Quadrupole MS + +-+ - Kinetic energy of an ion in an electrical field is proportional to its mass - Because of this, ions of lower m/z (m/z < 500, in this example) are accelerated more aggressively and eventually collide + - How do they select for an m/z? - Select for m/z 500 (positive) + Quadrupole MS + +- + - Kinetic energy of an ion in an electrical field is proportional to its mass - In contrast ions of higher m/z (m/z > 500, in this example) are slow to respond to the change in potential during the RF pulse + + +- How do they select for an m/z? - Select for m/z 500 (positive) + Quadrupole MS RF pulse - + + - Kinetic energy of an ion in an electrical field is proportional to its mass - In contrast ions of higher m/z (m/z > 500, in this example) are slow to respond to the change in potential during the RF pulse + + How do they select for an m/z? - Select for m/z 500 (positive) - - They are not refocused into the centre plane and are lost to the negative poles (DC potential) Quadrupole MS + +- + - Kinetic energy of an ion in an electrical field is proportional to its mass - In contrast ions of higher m/z (m/z > 500, in this example) are slow to respond to the change in potential during the RF pulse + +- How do they select for an m/z? - Select for m/z 500 (positive) + - They are not refocused into the centre plane and are lost to the negative poles (DC potential) Quadrupole MS How do they select for an m/z? + +- - Resolution of a quad is usually unity + +- - Only very specific m/z have their path appropriately corrected so that they pass safely through the quads - Can tell the difference between 499 and 500, but not between 499 and 499.5 + Triple Quadrupole MS Ion source Det Mass analyzer Q1 Collision Cell Q2 Mass analyzer Q3 Mass filter RF only All ions pass Mass filter Triple Quadrupole MS Positive ion in the collision cell - No mass analysis, all masses are allowed to pass + - + Collision Cell Q2 + + Parent ion 5-50 eV N2 Collision - + + Product ions - Collision cell is filled with an inert gas: Ar, He or N2. - Analyte is accelerated through the collision cell - Analyte collides with the inert gas - The faster this acceleration the more energetic the collision, and the more fragmentation - Process is called collisionallyinduced dissociation Triple Quadrupole MS Ion source Det Mass analyzer Q1 Parent ion selection + Collision Cell Q2 Collisionally-induced dissociation + N2 Mass analyzer Q3 Product ion selection + - + + + Triple Quadrupole MS Ion source Det Mass analyzer Q1 Parent ion selection Collision Cell Q2 Mass analyzer Q3 Collisionally-induced dissociation F F F Product ion selection F F F F F F F F F F F F O F F F F F F F O PFOA, 499 m/z F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F F Product, 369 m/z Fragments Triple Quadrupole MS What is the selling point of a quadrupole instrument - Cheap and small - Little downtime, workhorses What is the downside of a quadrupole instrument - Limited analyte information, usually only nominal mass analysis What is the selling point of a triple quadrupole instrument - The choice of two characteristic ions is very selective Selectivity lowers background noise (game of S:N) Very good sensitivity! Some structural information What is the downside of a triple quadrupole instrument - Can have some interferences and ‘false detections’