Document
advertisement

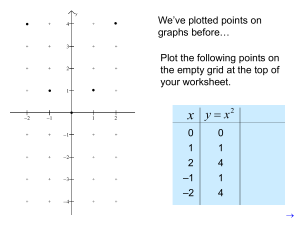
http://i249.photobucket.com/albums/gg225/ab17z/hump/33.jpg When Complete…. Stop and Think pg. 69 #1-6 Write out the Questions AND Answers in complete sentences. Work within your groups!!! Stop and Think pg 69, #1-6 1. Compare the highlight comments you wrote beside each “what I see” entry with others in your team. Modify your comments based on what you learned from others. Include those modifications in your notebook Constant positive slope Stop and Think pg 69, #1-6 2. Write “What it means” under each “what I see” comment. This phrase is the other half of your highlight comments. Explain what a constant positive slope means about the density of each liquid. Remember from math that constant slope means constant rise/run, for this graph, rise/run is the same as mass/volume if the line passes through the origin. Both ratios have the same numerical value What I See: Constant Positive Slope What it means: volume and mass are in a direct proportion for this liquid at room temperature and pressure Stop and Think pg 69, #1-6 3. Are the slopes of all three lines the same? What does that mean? Write your highlight comments about the relative sizes of slopes in a “What I see”, “What it means” format on the graph The slope for each liquid is different, yet each slope is constant. • That they are different means that the relationship between mass and volume is different for each liquid. • t means the density of each liquid is different. • And the density of a particular liquid is constant. Stop and Think pg 69, #1-6 4. Meet with your team and share what you have written for each comment on your graph. Decide as a team what to write as a caption under the graph. Think back to other graphs and charts you have read. Captions often told you, in sentence format, what you were looking at, what it meant, and why it was important. Include these features in your caption This graph shows how mass changes when volume increases for 3 different liquids. Notice how each liquid shows a steady increase in mass as its volume increases. Each data set closely follows a straight line. Since the slopes are different, the densities of each liquid are different. Even though the lines have different slopes for each liquid, the ratio between mass and volume for a given substance remains the same over all the measured values. This suggests that the relationship between mass and volume is constant for a given substance. Stop and Think pg 69, #1-6 5. Calculate the slopes of each best-fit line and show all your work with proper units and labels. • Slope is calculated as Rise/Run • Select two points from which you calculate the slope that lie on the Best-Fit Line NOT it does not have to be in the part of the original data set • If the line does not pass through the origin, do not use the origin as one of the points used in the slope calculation Stop and Think pg 69, #1-6 5. Calculate the slopes of each best-fit line and show all your work with proper units and labels. a) Find your calculated densities for each sample (you will have 9 calculations in your data table) D=M/V Remember to take the mass of only the liquid not the mass of the liquid AND graduated cylinder Stop and Think pg 69, #1-6 5. Calculate the slopes of each best-fit line and show all your work with proper units and labels. b) Compare those values with the slopes you just calculated You should see that your calculated sloes and average densities calculations for a given fluid are very close, within the expected variation in data due to instrument uncertainties Stop and Think pg 69, #1-6 5. Calculate the slopes of each best-fit line and show all your work with proper units and labels. c) What does it mean if they are very close to each other? What does it mean if they are very far apart? When the density and the slope are very close, it suggests that the slope and the density are the same. This is because Δy/Δx=y/x (calculation for slope)when it passes through the origin. What is on the y axis? What is on the x axis? What is the formula for density? If the densities are far apart, it suggests a procedural or calculation mistake Stop and Think pg 69, #1-6 6. Decide as a team the best way to explain how graphs help you “see” density differences Graphs help you “see” density because it is easy to see the steepness in slope. We cannot look at 2 clear liquids and tell by sight if the densities are the same. But with a graph, slope differences are very easy to see, especially for larger-sized samples. Sink or Float Part II: Determining the Density of a Solid TOC: Part II: Determining the Density of a Solid Work in your groups: Read Process and Procedures 1-2 Change in procedures: Instead of using water in your Graduated Cylinder use Alcohol. These graduated cylinders count by 2’s not 1’s Write up your procedures Create a table Read with your group on Stop and Think Part II on pg 71