Lecture 16
advertisement

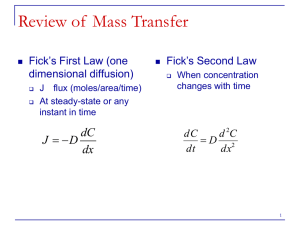
Kinetics III Lecture 16 Derivation of 5.67 • Begin with Ânet = Â+ (1- e∆ G/RT ) • Assume ∆G/RT is small so that e∆G/RT = 1+∆G/RT, then Ânet = -Â+ ∆ G / RT • Near equilibrium for constant ∆H, ∆S, ∆G = -(T-Teq)∆S Ânet = -Â+ -∆ S(T - Teq ) ∆ S(T - Teq ) ∆G = -Â+ = Â+ RTeq RTeq RTeq • Equation 5.67 should read: Ânet = o no negative, no square  + ∆ S(T - Teq ) RTeq ∆G & Complex Reactions • Our equation: Ânet = Â+ (1- e∆ G/RT ) • was derived for and applies only to elementary reactions. • However, a more general form of this equation also applies to overall reactions: Ânet = Â+ (1- en∆ G/RT ) • where n can be any real number. So a general form would be: Ânet = A+ e- E+ /RT (1- en∆ G/RT )anAA anBB ACnC ... Diffusion Importance of Diffusion • As we saw in the example of the N˚ + O2 reaction in a previous lecture, the first step in a reaction is bringing the reactants together. • In a gas, ave. molecular velocities can be calculated from the Maxwell-Boltzmann equation: v= 8kT pm • which works out to ~650 m/sec for the atmosphere • Bottom line: in a gas phase, reactants can come together easily. • In liquids, and even more so for solids, bringing the reactants together occurs through diffusion and can be the rate limiting step. Fick’s First Law • Written for 1 component and 1 dimension, Fick’s first Law is: ¶c J = -D ¶x o where J is the diffusion flux (mass or concentration per unit time per unit area) o ∂c/∂x is the concentration gradient and D is the diffusion coefficient that depends on, among other things, the nature of the medium and the component. • Fick’s Law says that the diffusion flux is proportional to the concentration gradient. A more general 3dimensional form (e.g., non-isotropic lattice) is: J = -DÑC æ D11 ç D = ç D21 ç D è 31 D12 D22 D32 D13 ö ÷ D23 ÷ D33 ÷ø Deriving Fick’s Law • On a microscopic scale, the mechanism of diffusion is the random motion of atoms. • Consider two adjacent lattice planes in a crystal spaced a distance dx apart. The number of atoms (of interest) at the first plane is n1 and at the second is n2. • We assume that atoms can randomly jump to an adjacent plane and that this occurs with an average frequency ν (i.e., 1 jump of distance dx every 1/ν sec) and that a jump in any direction has equal probability. • At the first plane there will be νn1/6 atoms that jump to the second plane (there are 6 possible jump directions). At the second plane there will be νn2/6 atoms that jump to first plane. The net flux from the first plane to the second is then: J= n n1 / 6 - n n2 / 6 dx 2 = n (n1 - n2 ) 6 dx 2 Deriving Fick’s Law J= n n1 / 6 - n n2 / 6 dx 2 = n (n1 - n2 ) 6 dx 2 • We’ll define concentration, c, as the number of atoms/unit volume n/x3, so: J == n (c1 - c2 )dx 3 6 dx 2 n = (c1 - c2 )dx 6 • Letting dc = -(c1 - c2) and multiplying by dx/dx J=- • Letting D= n dx 2 6 n dx 2 dc 6 dx then dc J = -D dx