c - Materials Science
advertisement

Chapter 5
Structure of
Solids
6 Lectures
1
Solids
Crystalline
Long-range periodicity
Gives sharp
diffraction patterns
Has sharp melting point
Has higher density
Noncrystalline
No long-range periodicity
Does not give sharp
diffraction patterns
Does not have a sharp
meliing point
Has a lower density
2
3
Factors promoting the formation of noncrystalline
structures
1. Primary bonds do not extend in all three
directions and the secondary bonds are not
strong enough.
2. The difference in the free energy of the
crystalline and non crystalline phases is
small.
3. The rate of cooling from the liquid state is
higher than a critical cooling rate.
Metallic Glass: 106 K s-1
4
Inorganic Solids
Covalent Solids
Metals and Alloys
Ionic Solids
Silica: crystalline and amorphous
Polymers
Classification
Structure
Crystallinity
Mechanical Behaviour
5
Inorganic Solids
Covalent Solids
Metals and Alloys
Ionic Solids
Silica: crystalline and amorphous
Polymers
Classification
Structure
Crystallinity
Mechanical Behaviour
6
Metals and Alloys
1. Metallic bond: Nondrectional (Fact)
As many bonds as geometrically possible (to
lower the energy)
Close packing
2. Atoms as hard sphere (Assumption)
3. Elements (identical atoms)
1, 2 & 3 Elemental metal crystals:
close packing of equal hard spheres
7
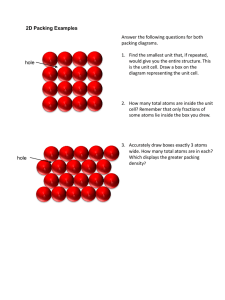
Close packing of equal hard spheres
Arrangement of equal nonoverlapping spheres
to fill space as densely as possible
Sphere packing problem:
What is the densest packing of spheres in 3D?
Kepler’s conjecture, 1611
P.E
3 2
0.74
Kissing Number Problem
What is the maximum number of spheres that
can touch a given sphere?
Coding Theory
Internet data transmission
8
Lecture 9
13.08.2013
9
We are currently preparing
students for jobs that do not
yet exist, using technologies
that haven’t been invented in
order to solve problems that we
don’t even know are problems
yet.
http://www.youtube.com/watch?v=XVQ1ULfQawk
10
Close packing of equal hard spheres
1-D packing
A chain of spheres
occupiedlength
P.E.=
=1
total length
Kissing Number= 2
Close-packed direction of atoms
11
Close packing of equal hard spheres
2-D packing
A hexagonal layer of atoms
Close-packed directions?
Close-packed plane of atoms
3
occupiedarea .907
P.E.=
total area
2 3
Kissing Number=6
1940 L. Fejes Toth : Densest packing of circles in plane
12
Close packing of equal hard spheres
3-D packing
First layer A
A
A
C
C
A
B
A
A
A
B
B
A
B
C
C
Third layer A or C
A
C
C
B
Second layer B
B
A
A
A
C
C
B
B
A
A
A
C
B
A
A
Close packed crystals:
…ABABAB… Hexagonal close packed (HCP)
…ABCABC… Cubic close packed (CCP)
13
Geometrical properties of ABAB stacking
A
C
B
a
b=a
A
=120
C
A
B
B
C
B
A
A
A
c
B
A
C
B
B
A
B
C
A
C
C
A
C
A
A
A
B
A
B
A
A
C
B
A
A
A and B do not have identical neighbours
Either A or B as lattice points, not both
Unit cell: a rhombus based prism with a=bc; ==90, =120
The unit cell contains only one lattice point (simple) but two atoms (motif)
ABAB stacking = HCP crystal = Hexagonal P lattice + 2 atom motif 000
14
2/3 1/3 1/2
z
Hexagonal close-packed (HCP) crystal
½
y
½
½
½
Corner and inside atoms do not have
the same neighbourhood
x
Lattice: Simple hexagonal
hcp lattice
Motif: Two atoms:
000; 2/3 1/3 1/2
hcp crystal
16
c/a ratio of an ideal HCP crystal
A
A
C
C
B
B
C
B
A
B
A
C
B
A
c
C
A
B
A
B
C
A
B
C
A
C
A
A
A
B
A
A
A
C
B
A
B
A
A
A single B atom sitting on a base of three A atoms forms a regular
tetrahedron with edge length a = 2R
The same B atom also forms an inverted tetrahedron with three A atoms
sitting above it
c = 2 height of a tetrahedron of edge length a
2 2
c
a
3
17
Lec 10: Structure of metals and alloys (Close
Packing) continued
16.08.2013
Rescheduled Lecture for the missed lecture on
Wednesday: Today 7-8 pm VLT1
Office hours for discussions:
5-6 pm on tuesdays, wednesdays and
thursdays
Doubt clearning class on request
18
Geometrical properties of ABCABC stacking
A
A
C
C
A
B
A
A
B
A
B
C
C
B
A
C
C
B
A
B
A
A
A
C
C
B
B
A
A
A
C
B
A
A
19
Geometrical properties of ABCABC stacking
C
B
All atoms are equivalent
A
and their centres form a
C
lattice
3 a
B
A
Motif: single atom 000
What is the Bravais
lattice?
ABCABC stacking
= CCP crystal
= FCC lattice + single atom motif 000
20
Close packed planes in the FCC unit cell of cubic close
packed crystal
Body
diagonal
A
B
C
A
Close packed planes: {1 1 1}
21
Stacking
sequence?
22
Stacking
sequence?
23
Find the mistake in the following figure from a
website:
http://www.tiem.utk.edu/~gross/bioed/webmodules/s
pherefig1.gif
24
Table 5.1
Coordination Number and Packing Efficiency
CW
Crystal
Structure
Diamond cubic (DC)
Coordination
number
4
HW
Packing
efficiency
0.32
0.52
Simple cubic (SC)
6
Body-centred cubic
8
0.68
Face-centred cubic
12
0.74
Empty spaces are distributed in various
voids
25
End of lecture 10 (16.08.2013)
Beginning of lecture 11
26
Voids in Close-Packed Crystals
TETRAHEDRAL VOID
OCTAHEDRAL VOID
B
A
A
A
B
C
A
A
B
A
No. of atoms defining
the void
4
No. of voids per atom
2
Edge length of void
2R
Size of the void
0.225 R
B
A
A
6
A
Experiment 2
1
2R
HW
0.414 R
27
Locate of Voids in CCP Unit cell
28
Solid Solution
A single crystalline phase consisting of two or more elements is
called a solid solution.
Substitutional Solid solution
of Cu and Zn (FCC)
Interstitial solid solution of
C in Fe (BCC)
29
Hume-Rothery Rules for Extensive
Solid Solution (Unlimited solubility)
Interstitial solid solution
Substitutional solid solution
1. Structure factor
Crystal structure of the two elements should be
the same
2. Size factor:
Size of the two elements should not differ
by more than 15%
3. Electronegativity factor:
Electronegativity difference between the elements
should be small
4. Valency factor:
Valency of the two elements should be the same
30
TABLE 5.2
System
Crystal
structure
Radius of
atoms, Ǻ
Valency
Electronegativity
Ag-Cu
Ag
Au
FCC
FCC
1.44
1.44
1
1
1.9
1.9
Cu-Ni
Cu
Ni
FCC
FCC
1.28
1.25
1
2
1.9
1.8
Ge-Si
Ge
Si
DC
DC
1.22
1.18
4
4
1.8
1.8
All three systems exhibit complete solid solubility.
31
BRASS
Cu
FCC
+
Zn
HCP
Unfavourable structure factor
Limited Solubility:
Max solubility of Cu in Zn: 1 wt% Cu
Max Solubility of Zn in Cu: 35 wt% Zn
32
Ordered and Random
Substitutional solid solution
Random Solid
Solution
Ordered Solid
Solution
33
Ordered and random substitutional solid
solution
β-Brass: (50 at% Zn, 50 at% Cu)
Disordered solid solution of β-Brass:
Above
470˚C
470˚C
Below
470˚C
Corner and centre both have
50% proibability of being
occupied by Cu or Zn34
Ordered solid solution of β-Brass:
Corners are always occupied
by Cu, centres always by Zn
34
Intermediate Structures
Crystal structure of Cu:
FCC
Crystal structure of Zn:
HCP
Crystal structure of
random β-brass:
BCC
Such phases that have a crystal structure different
from either of the two components are called
INTERMEDIATE STRUCURES
If an intermediate structure occurs only at a fixed
composition it is called an INTERMETALLIC
COMPOUND, e.g. Fe3C in steels.
35
End of lecture 10 (16.08.2013)
Beginning of lecture 11
36
4th. Group: Carbon
37
Allotropes of C
Graphite
Buckminster Fullerene
1985
Diamond
Carbon Nanotubes
1991
Graphene
2004 38
Graphite
Sp2 hybridization 3 covalent bonds
Hexagonal sheets
a = 2 d cos 30°
= √3 d
y
x
a
=120
b=a
d = 1.42 Å
a = 2.46 Å
39
Graphite
a = 2.46 Å
c = 6.70 Å
Lattice: Simple Hexagonal
Motif: 4 carbon atoms
000; 2/3 1/3 0; 2/3 1/3 1/2; 1/3 2/3 1/2
A
x
B
y
A
www.scifun.ed.ac.uk/
40
c
Graphite
Highly Anisotropic:
Properties are very different in the a and c directio
Uses:
Solid lubricant
Pencils (clay + graphite, hardness
depends on fraction of clay)
carbon fibre
www.sciencemuseum.org.uk/
41
Diamond
Sp3 hybridization 4 covalent bonds
Tetrahedral bonding
Location of atoms:
8 Corners
6 face centres
4 one on each of the 4 body diagonals
42
Diamond Cubic Crystal: yLattice1 & motif?
0,1
M
S
P
N
D
A
T
B
3
4
Q
L
K
D
y
R
C
1
2
R
M
S
A
0,1
C
1
4
L
Q
T
0,1
1
4
x
0,1
2
K
3
4
1
2
N
B
P
1
2
0,1
x
Projection of the unit cell on
the bottom face of the cube
Diamond Cubic Crystal
= FCC lattice + motif: 000; ¼¼¼
43
Crystal Structure = Lattice + Motif
Diamond Cubic
Crystal Structure
=
FCC
Lattice
+
2 atom
Motif
000
1 1 1
4 4 4
There are only three Bravais Lattices: SC, BCC, FCC.
Diamond Cubic
Lattice
44
There is no
diamond
cubic
lattice.
45
Diamond Cubic
Structure
Coordination 4
number
Corners
Face
Inside
1
1
8 6 1 4 8
Effective number of atoms in the unit cell =
8
2
Relaton between lattice parameter
and atomic radius
3a
2r
4
8r
a
3
Packing efficiency
4 3
8 r
3
3
0.34 46
3
a
16
Diamond Cubic Crystal Structures
C
Si
Ge
Gray Sn
a (Å) 3.57 5.43 5.65 6.46
47
Equiatomic binary AB compounds having
diamond cubic
y
1
2
0,1
0,1
like structure
IV-IV compound: SiC
III-V compound:
AlP, AlAs, AlSb,
GaP, GaAs, GaSb,
InP, InAs, InSb
1
4
3
4
1
2
S
1
2
0,1
1
4
1
2
3
4
II-VI compound:
ZnO, ZnS,
CdS, CdSe, CdTe
I-VII compound:
CuCl, AgI
0,1
0,1
48
USES:
Diamond
Abrasive in polishing and grinding
wire drawing dies
Si, Ge, compounds:
semiconducting devices
SiC
abrasives, heating elements of furnaces
49
End of lecture 11 (16.08.2013)
(Evening class, a postponed class for the missed
class on Wednesday 14.08.2013)
Beginning of lecture 12
50
Allotropes of C
Graphite
Buckminster Fullerene
1985
Diamond
Carbon Nanotubes
1991
Graphene
2004
C60 Buckminsterfullerene
H.W. Kroto, J.R. Heath, S.C. O’Brien, R.F. Curl and R.E.
Smalley
Nature 318 (1985) 162-163
Long-chain carbon molecules
in interstellar space
1996 Nobel Prize
A carbon atom at each
vertex
American architect,
author, designer,
futurist, inventor, and
visionary.
He was expelled from Harvard twice:
1. first for spending all his money partying with a
Vaudeville troupe,
2. for his "irresponsibility and lack of interest".
what he, as an individual, could do to improve humanity's
condition, which large organizations, governments, and
private enterprises inherently could not do.
Montreal Biosphere in Montreal, Canada
Truncated Icosahedron
Icosahedron: A Platonic solid (a regular solid)
Truncated Icosahedron: An Archimedean solid
A regular polygon
A polygon with all sides equal and all angles equal
Square
regular
Rectangle
unequal sides
not regular
Rhombus
unequal angles
not regular
Regular Polygons: All sides equal all angles equal
Triangle square
3
4
pentagon
hexagon…
5
6…
A regular n-gon with any n >= 3 is possible
There are infinitely many regular polygons
3D: Regular Polyhedra or Platonic Solids
All faces regular congruent polygons, all corners
identical.
Cube
How many regular solids?
Tetrahedron
There are 5
and only 5
Platonic or
regular solids !
59
There are 5
and only 5
Platonic or
regular
solids !
V
E
F
1. Tetrahedron
4
6
4
2. Octahedron
6
12
8
3. Cube
8
12
6
4. Icosahedron
12
30
20
5. Dodecahedron
20
30
12
Duals
Duals
Euler’s
Polyhedr
on
Formula
V-E+F=2
Duality
Tetrahedron
Self-Dual
Octahedron-Cube
Icosahedron-Dodecahedron
Proof of Five Platonic Solids
At any vertex at least three faces should meet
The sum of polygonal angles at any vertex should
be less the 360
Triangles (60)
3
Tetrahedron
4
Octahedron
5
Icosahedron
6 or more: not possible
Square (90)
3
Cube
4 or more: not possible
Pentagon (108)
3
Dodecahedron
Truncated Icosahedron: V=60, E=90, F=32
Nature 391, 59-62 (1
January 1998)
Electronic structure of
atomically resolved
carbon nanotubes
Jeroen W. G. Wilder,
Liesbeth C. Venema,
Andrew G. Rinzler,
Richard E. Smalley &
Cees Dekker
Structural features of carbon nanotubes
a
a
2
1
=chiral angle
zigzig
(n,0)
(n,m)=(6,
3)
Material
Young's
Modulus (TPa)
Tensile
Strength
(GPa)
SWNT
~1 (from 1 to 5)
13-53
Armchair
SWNT
0.94
Zigzag
SWNT
0.94
Chiral
SWNT
0.92
MWNT
0.8-0.9
150
Stainless
Steel
~0.2
~0.65-1
15-50
Kevlar
~0.15
~3.5
~2
0.25
29.6
Kevlar
T
T
126.2
T
94.5
E
E
T
T
Elongation at
Break (%)
16
23.1
15.6-17.5
Source: wiki
Electrical
For a given (n,m) nanotube, if n = m, the nanotube is
metallic;
if n − m is a multiple of 3, then the nanotube is
semiconducting with a very small band gap,
otherwise the nanotube is a moderate semiconductor.
Thus all armchair (n=m) nanotubes are metallic,
and nanotubes (5,0), (6,4), (9,1), etc. are semiconducting.
In theory, metallic nanotubes can carry an electrical
current density of 4×109 A/cm2 which is more than 1,000
times greater than metals such as copper[23].
While the fantasy of a space elevator has
been around for about 100 years, the idea
became slightly more realistic by the 1991
discovery of "carbon nanotubes,"
The Space Engineering and Science Institute
presents
The 2009 Space Elevator Conference
Pioneer the next frontier this summer with a four-day
conference on the Space Elevator in Redmond, Washington
at the Microsoft Conference Center.
Thursday, August 13 through Sunday, August 16, 2009
Register Today!
End of lecture 12 (20.08.2013)
Beginning of lecture 13 (21.08.2013)
73
IONIC SOLIDS
Cation radius: R+
Anion radius: RUsually
R R
1. Cation and anion attract each other.
2. Cation and anion spheres touch each other
3. Ionic bonds are non-directional
1, 2, 3 => Close packing of unequal spheres
74
IONIC SOLIDS
Local packing geometry
1. Anions and cations considered as hard spheres
always touch each other.
2. Anions generally will not touch, but may be close
enough to be in contact with each other in a
limiting situation.
3. As many anions as possible surround a central cation
for the maximum reduction in electrostatic
energy.
75
Effect of radius ratio
Rc
0.155
Ra
unstable
Anions not touching the
central cation,
Anions touching each
other
Rc
0.155
Ra
Rc
0.155
Ra
Critically stable
stable
Anions touching
the central
cation
Anions touching
Rc
0.155 Ligancy 2
Ra
Anions touching
central cation
Anions not touching
each other
Rc
0.155 Ligancy 3
Ra
76
Rc
0.155 Ligancy 3
Ra
However, when
Rc
0.225
Ra
tetrahedral coordination
with ligancy 4 becomes stable
Recall tetrahedral void in close-packed
structure.
Thus
Rc
0.155
0.225 Ligancy 3
Ra
77
Table 5.3
Ligancy as a Function of Radius Ration
Ligancy
Range of radius ratio
2
0.0 ― 0.155
3
0.155 ― 0.225
Triangular
4
0.225 ― 0.414
Tetrahedral
6
0.414 ― 0.732
Octahedral
8
0.732 ― 1.0
Cubic
12
1.0
Configuration
Linear
FCC or HCP
78
Example 1:
NaCl
RNa
RCl
0.54
0.414 0.54 0.732
Ligancy6
OctahedralCoordination
cae2k.com
NaCl structure =FCC lattice + 2 atom motif: Cl- 0 0 0
Na ½ 0 0
79
NaCl structure
continued
CCP of Cl─ with Na+ in ALL octahedral voids
2RNa" 2RCl a
80
Example 2 : CsCl Structure
RCs
RCl
0.91
0.732 0.91 1
Ligancy 8
Cubic coordination of Claround Cs+
seas.upenn.edu
CsCl structure = SC lattice + 2 atom motif: Cl 000
Cs ½ ½ ½
BCC
2RCs 2RCl 3a
81
Example 3: CaF2 (Fluorite or fluorospar)
RCa 2
RF
0.73
0.73 0.732
Octahedral or cubic
coordination
Actually cubic coordination
of F─ around Ca2+
But the ratio of number of F─ to Ca2+ is 2:1
So only alternate cubes of F─ are filled with Ca2+
82
Simple cubic crystal of F─ with Ca2+ in alternate cube centres
Alternately, Ca2+ at
FCC sites with F─ in
ALL tetrahedral voids
CaF structure= FCC lattice + 3 atom motif
Ca2+
F─
F─
000
¼¼¼
-¼ -¼ -¼
83
Example 4: ZnS (Zinc blende or sphalerite)
RZn 2
RS 2
0.48
0.414 0.48 0.732
Ligancy6
OctahedralCoordination
However, actual ligancy is 4
(TETRAHEDRAL
COORDINATION)
wikipedia
Explanation: nature of bond is more covalent than ionic
84
ZnS structure
CCP of S2─ with Zn2+ in alternate tetrahedral voids
seas.upenn.edu
ZnS structure = FCC lattice + 2 atom motif
S2─ 0 0 0
Zn2+ ¼ ¼ ¼
85
86
pixdaus.com
87
theoasisxpress
88
What is common to
1, glass of the window
2. sand of the beach, and
3. quartz of the watch?
89
pixdaus.com
Structure of SiO2
Bond is 50% ionic and 50 % covalent
RSi 4
RO 2
0.29
0.225 0.29 .414
Tetrahedral coordination of
O2─ around Si4+
Silicate
tetrahedron
90
Silicate tetrahedron electrically
unbalanced
4─
2─
4+
2─
2─
2─
O2─ need to be shared between two tetrahedra
91
1. O2─ need to be shared between two tetrahedra.
2. Si need to be as far apart as possible
Face sharing
Edge sharing
Corner sharing
Silicate tetrahedra share corners
92
2D representation of 3D periodically repeating pattern of
tetrahedra in crystalline SiO2. Note that alternate tetrahedra
93
are inverted
2 D representation of 3D random network of silicate
tetrahedra in the fused silica glass
94
Network Modification by addition of Soda
Na
+ Na2O =
Na
Modification leads to breaking of primary bonds between
silicat tetrahedra.
95
2 D representation of 3D random network of silicate
tetrahedra in the fused silica glass
96
End of lecture 13 (21.08.2013)
Syllabus for minor I (upto lecture 13)
Beginning of lecture 14 (23.08 2013)
97
5.7 Structure of Long Chain
Polymers 109.5
H
Degree of
Polymerization:
No. of repeating
monomers in a chain
H
C
A
C
C
98
Freedom of rotation about each bond in space
leads to different conformations of C-C
backbone
109.5
99
100
5.8 Crystallinity in long chain polymers
Fig. 5.17: semicrystalline polymer
101
Factors affecting crystallinity of a long chain
polymer
1. Higher the degree of polymerization lower is
the degree of crystallization.
Longer chains get easily entagled
102
Branching
2. More is the branching less is the tendency to
crystallize
103
Tacticity
3. Isotactic and syndiotactic polymers can
crystallize but atactic cannot.
104
Copoymers: polymeric analog of solid
solutions
4. Block and random copolymers promote non
crystallinity.
105
Plasticizers
Low molecular weight additives
Impedes chains coming together
Reduces crystallization
106
Elastomer
Polymers with very extensive elastic deformation
Stress-strain relationship is non-linear
Example: Rubber
107
Liquid
natural
rubber
(latex)
being
collected
from the
rubber
tree
108
Isoprene molecule
commons.wikimedia.org
H H3C H
C=C-C=C
HH
H
109
H
H
CH3 H
H
Isoprene
molecule
CH3 H
CCCC
H
H
CCCC
H
H
H
Polymerization
Polyisoprene
mer
Liquid
(Latex)
110
Vulcanisation
H
H
CH3 H
CCCC
H
H
+ 2S
H
H
CCCC
H H CH H
Weak
van der
Waals
bond
3
111
Vulcanisation
H
H
CH3 H
CCCC
H
H
S S
H
H
Crosslinks
CCCC
H H CH H
3
112
Effect of cross-linking on polyisoprene
Natural
rubber
Elastomer
Ebonite
liquid
Elastic
solid
Hard &
brittle
not
x-linked
lightly
x-linked
heavily
x-linked
113
Charles Goodyear
December 29, 1800-July 1, 1860
Debt at the time of
death $200,000
Life should not be estimated
exclusively by the standard of dollars
and cents. I am not disposed to
complain that I have planted and
others have gathered the fruits. One
has cause to regret only when he
sows and no one reaps.
114
End of lecture 14 (23.08.2013)
Beginning of Lecture 15 (27.08.2013)
(rubber elasticity + pre minor I discussion)
115
Another interesting property of
elastomers
Thermal behaviour
116
Elastomer sample
under tension
Elastomer
sample
heat
Tensile force
Coiled chains
Higher
entropy
straight
chains
Lower
entropy
Contracts on
heating
Still
lower
entropy
F
117
Elastomers have ve thermal
expansion coefficient, i.e., they
CONTRACT on heating!!
EXPERIMENT 4
Section 10.3 of the textbook
118
N 0 kT
F
L0
F
N0
k
T
L0
L
L L0
L0 L
2
applied tensile force
number of cross-links
Boltzmann constant
absolute temperature
initial length (without F)
final length (with F)
119
Bond stretching in straightened
out molecules
Experimental
Theory: Chain uncoiling
N kT
F 0
L0
L L0 2
L0 L
120