H 6 - Gas exchange - IBDPBiology-Dnl
advertisement

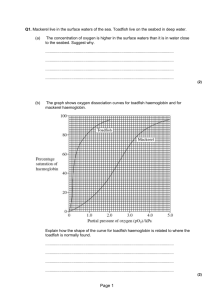
Assessment Statements H.6.1 H.6.2 Define partial pressure. Explain the oxygen dissociation curves of adult hemoglobin, fetal hemoglobin and myoglobin. H.6.3 Describe how carbon dioxide is carried by the blood, including the action of carbonic anhydrase, the chloride shift and buffering by plasma proteins. H.6.4 Explain the role of the Bohr shift in the supply of oxygen to respiring tissues. H.6.5 Explain how and why ventilation rate varies with exercise. H.6.6 Outline the possible causes of asthma and its effects on the gas exchange system. H.6.7 Explain the problem of gas exchange at high altitudes and the way the body acclimatizes. Define the term partial pressure partial pressure is the pressure exerted by a given gas in a mixture the symbol for partial pressure is P, and the partial pressure for a gas x is Px. So, PO2 denotes the partial pressure of oxygen what is the partial pressure of the oxygen in the air Atmospheric air is a mixture of gases; around us? nitrogen, oxygen, carbon dioxide, water At sea level, the atmospheric vapour & inert gases. pressure is typically about At sea level, the atmospheric pressure 101.3 kPa of which 21.0% is is about 101.3 kPa. O2, So, PO2 is given by: What proportion of atmospheric 101.3 𝑥 21.0 pressure is due to oxygen? = 21.3 kPa 100 Role of haemoglobin 1 molecule of oxygen will combine with each haem group, meaning, each haemoglobin molecule is able to transport 4 molecules of oxygen: oxyhaemoglobin is the form in which oxygen is transported from the lungs to the respiring haemoglobin occurs in the body tissues red cells at respiring tissue cells, haemoglobin molecule is oxyhaemoglobin breaks down, built of four interlocking releasing oxygen & haemoglobin subunits each subunit is composed of a oxygen is used up by tissue cells while haemoglobin is returned large globular protein with to the lungs to pick up more a non-protein haem group oxygen attached, containing iron Oxygen dissociation curve the affinity of haemoglobin for oxygen is measured experimentally by finding the percentage saturation with oxygen of blood exposed to air mixtures containing different partial pressures of oxygen the result is called an oxygen dissociation curve oxygen dissociation curve is S-shaped, the amount of oxygen held by haemoglobin depends on the partial pressure of oxygen in the body, too, the amount of oxygen held by haemoglobin depends on the partial pressure in respiring tissues, the oxygen partial pressure is much lower than that in the lungs at lower partial pressures, oxyhaemoglobin breaks down, releasing oxygen in solution and this rapidly diffuses into the surrounding tissues Oxygen dissociation curve of adult haemoglobin oxygen dissociation curve for oxyhaemoglobin is S/sigmoid-shaped it shows how the saturation of Shift to the right; (decreased affinity) low pH, increased CO2, increased lactic acid haemoglobin with oxygen varies with partial pressure of oxygen haemoglobin has an increasing affinity for oxygen, initial uptake of one oxygen molecule by haemoglobin facilitates the further uptake of oxygen molecules low partial pressure of oxygen corresponds to the situation in the tissue, when partial pressure of oxygen is low, oxygen is released low pH, increased carbon dioxide & increased lactic acid causes the curve to shifts the to the right and oxygen is more readily released to respiring tissues – this is known as the Bohr effect high partial pressure of oxygen corresponds to the situation in the lungs, when partial pressure of oxygen is high, oxygen is taken up by haemoglobin Oxygen dissociation curve of fetal haemoglobin like adult haemoglobin, fetal haemoglobin between foetal & adult haemoglobin, which one has a higher affinity for oxygen? why it is advantageous that fetal haemoglobin higher affinity for oxygen than adult haemoglobin? have S-shaped oxygen dissociation curves fetal haemoglobin have a high affinity for oxygen at high partial pressure of oxygen fetal haemoglobin always has a higher affinity for oxygen at corresponding partial pressures of oxygen than adult haemoglobin, thus fetal haemoglobin dissociation curve lies to the left of the adult dissociation curve in the placenta where maternal and fetal blood come into close proximity there is a low oxygen partial pressure fetal haemoglobin must have a greater affinity for oxygen otherwise the maternal oxy-haemoglobin would not dissociate relationship between fetal and adult haemoglobin dissociation curves does NOT change at all partial pressures of oxygen the difference in adult and fetal haemoglobin structures lead to differences in affinity Oxygen dissociation curve of myoglobin myoglobin is specialized for oxygen myoglobin is a respiratory pigment built of a single haem–globin unit, similar to the four units in haemoglobin myoglobin is only found in skeletal muscle cells, where it acts as a reserve of oxygen storage myoglobin has a higher affinity for oxygen than haemoglobin, its dissociation curve is to the left of that for haemoglobin in normal conditions, at rest myoglobin is saturated with oxygen myoglobin is used during intense muscle contraction when the oxygen supply is insufficient i.e. when muscle is very active its oxygen concentration may fall below 0.5 kPa when this happens myoglobin releases oxygen to muscle cells myoglobin oxygen dissociation curve is not sigmoid shaped, it has a steep rise below 5 kPa with no lag & has slower rise approaching 100 % above 5 kPa Oxygen dissociation curves of adult haemoglobin, fetal haemoglobin and myoglobin adult haemoglobin: rapid saturation of oxygen in the lungs rapid dissociation of oxygen as the oxygen concentration decreases oxygen released in the tissues where it is needed fetal haemoglobin: fetal haemoglobin curve to the left of adult haemoglobin higher affinity for oxygen than adult haemoglobin oxygen moves from adult haemoglobin to fetal haemoglobin myoglobin: myoglobin to the left of fetal haemoglobin higher affinity for oxygen than adult haemoglobin only releases oxygen at very low oxygen concentrations in tissues acts as oxygen reserve in muscle cells How carbon dioxide is carried by the blood carbon dioxide is carried in three forms in the blood: carbon dioxide can be dissolved in the blood plasma forming carbonic acid (5 %) carbon dioxide can be carried as dissociated carbonic acid i.e. H+ + H CO3 − in red blood cells (85 %) carbon dioxide can be carried as carbaminohemoglobin when it is bound to haemoglobin (10 %) carbonic anhydrase is an enzyme found in red blood cells (erythrocytes) carbonic anhydrase speeds up production of hydrogen carbonate (H CO3 −) chloride shift i.e. movement of chloride ions into red blood cell, occurs to balance movement of hydrogen carbonate ion out Role of the Bohr shift in the supply of oxygen to respiring tissues hemoglobin carries up to four oxygen molecules Bohr shift promotes the release of oxygen in respiring heart muscle active respiration releases CO2 causing the partial pressure of CO2 increases release of CO2 increases acidity i.e. lowers the pH due to formation of hydrogen ions (H+) hydrogen ions bind to hemoglobin decreasing hemoglobin’s affinity for O2 so O2 is released from the oxyhemoglobin this occurs due allosteric effect i.e. conformational change in hemoglobin which releases O2 more readily Bohr shift How and why ventilation rate varies with exercise during exercise the rate of tissue respiration increases i.e. more carbon dioxide produced carbon dioxide production in the tissues exceeds the rate of breathing it out increase in carbonic acid (H2CO3), increase in H+ ions , pH drops in the blood plasma lactic acid produced during strenuous exercise reduces pH chemoreceptors, located in the carotid & aortic bodies, detect change in pH, increase in carbon dioxide & decrease in oxygen Increased CO2 in the blood & lower pH are also detected by chemoreceptors in medulla nerve impulses sent to the breathing Centre in the medulla of the brain from the chemoreceptors nerve impulses are then sent to diaphragm & intercostal muscles from the breathing Centre in medulla to increase the rate & the depth of breathing ventilation rate is controlled through negative feedback mechanism Possible causes of asthma and its effects on the gas exchange system asthma is a chronic inflammatory disease of the airway it is caused by allergic reaction to allergens such as; dust, mites droppings, pollen, toxins, pets hairs, fungi etc. immune responses releases histamine which causes: constriction of muscles of wall of bronchioles more mucus is produced these restricts air flow thus ventilation is hard & gas exchange is reduced Problem of gas exchange at high altitudes at high altitudes partial pressure of oxygen is lower, at 7000m PO2 is 8.1 kPa as air is exchanged in lungs hemoglobin does not become fully saturated with O2 oxygen deprivation of tissues occurs causing fatigue i.e. Monge disease mountain sickness (increased pulse rate, nausea, headaches, sore throat, muscular weakness, dizziness ) may develop ventilation rate & depth increases How the body acclimatizes to high altitudes ventilation rate increases red blood cell (erythrocyte) concentration in blood increases myoglobin concentration in muscles increases capillary networks in the muscles develop greater density lung working volume, vital capacity, increases people living permanently at high altitude develops greater lung surface area Revision Questions Define the term partial Explain why ventilation rate pressure. [1] Explain the oxygen dissociation curves of adult haemoglobin, fetal haemoglobin and myoglobin. [6] Describe how carbon dioxide is carried by the blood. [4] Explain, with the use of a diagram, the role of the Bohr shift in the supply of oxygen to respiring heart muscle. [6] Explain the Bohr shift of an oxygen dissociation curve during gas exchange. [6] varies with exercise. [6] Explain how and why ventilation rate varies with exercise. [6] Outline one possible cause of asthma and its effect on the gas exchange system. [3] Outline how the body acclimatizes to high altitudes. [3] Explain the problem of gas exchange at high altitudes and the way the body acclimatizes. [6] The oxygen dissociation curve is a graph that shows the percentage saturation of haemoglobin at various partial pressures of oxygen. Curve A shows the dissociation at a pH of 7 and curve B shows the dissociation at a different pH. (i) State the possible cause of the curve shifting from A to B. [1] (ii) On the graph, draw the curve for myoglobin. [2] Explain the oxygen dissociation of myoglobin, completing the graph below to support your answer. Po2 is the partial pressure of oxygen.