Lecture 10
advertisement
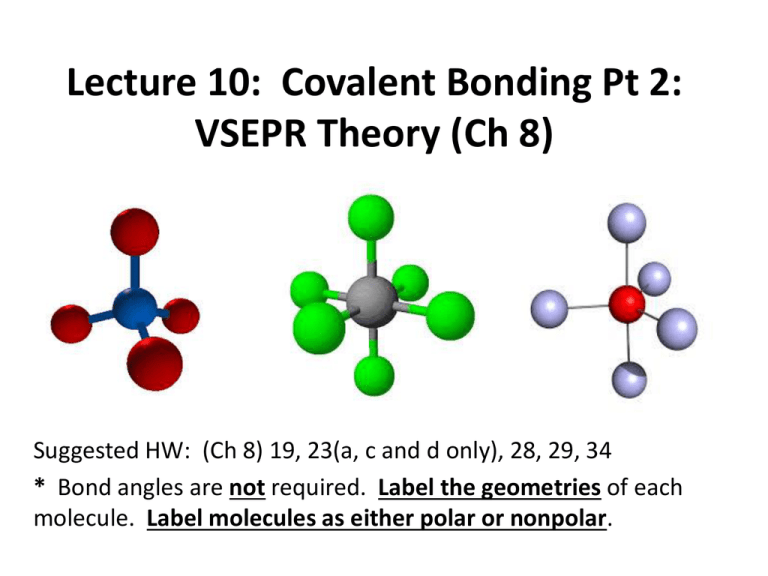
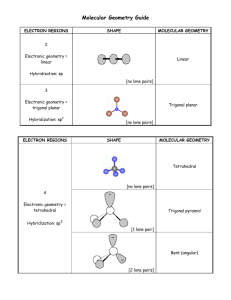
Lecture 10: Covalent Bonding Pt 2: VSEPR Theory (Ch 8) Suggested HW: (Ch 8) 19, 23(a, c and d only), 28, 29, 34 * Bond angles are not required. Label the geometries of each molecule. Label molecules as either polar or nonpolar. Introduction To date, we have learned about the Lewis structures of covalent bonds Lewis structures give insight into how atoms are bonded within a molecule, but does NOT tell us about the shape (molecular geometry), of the molecule Molecular geometry plays a major role in the properties of a substance. Considering Molecular Geometry If we draw the Lewis structures of water without considering geometry, we would derive the following: δ+ H δ- O δ+ H • The Lewis structure suggests that water is a linear (straight) molecule. • However, if this were true, then the dipoles moments would be in opposite directions, as described above, and water would be a nonpolar molecule If this were the case, life as we know it would be very different Considering Molecular Geometry The actual geometry of water is shown below: δδ+ H O 104.5o + H = δ+ • This is a bent geometry. The angle between the atoms is 104.5o. In this geometry, the molecule has a net dipole moment directed upward, which is why water is polar. • How do we determine the geometry? VSEPR Theory • The images below show balloons tied together at their ends. • There is an optimum geometry for each number of balloons, and the balloons spontaneously attain the lowest-energy arrangement. • In other words, the balloons try to “get out of each other’s way” as best they can. These arrangements maximize the distance between the balloon centers. Electrons behave the same exact way. VSEPR • In the valence-shell electron-pair repulsion theory (VSEPR), the electron domains around a central atom: – are arranged as far apart from each other as possible – have the least amount of repulsion of the negatively charged electrons – have a geometry around the central atom that determines molecular shape Using VSEPR To Predict Geometry STEP 1 Figure out the Lewis dot structure of the molecule. Determining Polarity Now that we know the geometry of molecules, we can determine whether or not the molecule is polar (has an overall dipole moment) • A polar molecule – contains polar bonds, as determined from differences in electronegativity (lecture 14) – has a separation of positive and negative partial charges, called a dipole, indicated with + and – – has dipoles that do not cancel (not symmetrical) Nonpolar Molecules • A nonpolar molecule – contains nonpolar bonds, as determined from differences in electronegativity – Or may be symmetrical – Or dipoles cancel Cl + δ H Cl HH Hδ+ δC H + δ δ -O δ + C O δ OVERALL DIPOLE = 0 H + δ Symmetrical Total Electron Domains 2 Domain Geometry Bonding Lone Pair Around Domains Domains Central Atom 2 MOLECULAR GEOMETRY A B 0 B Linear Ex. CO2 B 3 0 Trigonal planar A B 3 B Ex. BH3 Bent 104.5o •• A 2 1 B B Ex. NO2- Just a note Any molecule containing only two atoms must be linear. There is no other possible arrangement. Ex. H2, HCl, CO, etc. Examples Give the Lewis structures and geometries of the following molecules. Label each as polar or nonpolar: SO3 SO2 F2 4-Coordinate Molecules Have a Tetrahedral Arrangement • A tetrahedron is a shape consisting of 4 triangular faces. The vertices are separated by an angle of 109.5o, and each position is equivalent. • Another way to view a tetrahedron is to imagine a cube with atoms at opposite corners, with the central atom at the center of the cube. Total Electron Domains Domain Geometry Bonding Lone Pair Around Domains Domains Central Atom MOLECULAR GEOMETRY B 4 0 Tetrahedral A B B B Ex. CH4 •• 4 3 1 A B Trigonal Pyramidal B B Ex. NH3 •• Bent A 104.5o B 2 2 B Ex. H2O Examples Give the chemical structures and geometries of the following molecules. Label each as polar or nonpolar. SO42 PF3 OF2 CCl4 Expanded Electron Domains As stated in the previous lecture, central atoms with a principal quantum number of n>3 can accommodate more than 8 valence electrons. In many instances, there will be 5 or 6 bonds around these central atoms The regions occupied by the constituent atoms in a 5-coordinate structure are not equivalent. The constituent atoms may be either equatorial or axial. Five-Coordinate Molecules Z X Axial position (z axis) Equatorial position (x-y plane) Y • Five coordinate molecules assume some variation of the trigonal bipyramidal configuration shown to the right. • If you have a 5 coordinate molecule which contains a lone pair, like SF4, the lone pair will go in an equatorial position. LONE PAIR WANT TO BE AS FAR AWAY FROM OTHER ELECTRON DOMAINS AS POSSIBLE, AND SHOULD ALWAYS BE PLACED IN EQUITORIAL POSITIONS !!!! Total Electron Domains Domain Geometry Bonding Lone Pair Around Domains Domains Central Atom MOLECULAR GEOMETRY B 5 0 B B A B B 5 4 1 B B 2 B Ex. PCl5 Seesaw A B 3 Trigonal Bipyramidal A B B Ex. SF4 T-shaped B Ex. ClF3 A Five-Coordinate molecule with 3 Lone pairs is LINEAR Symmetrical about the central atom. Ex. XeF2 Note: In this chapter, you will find examples of noble gases acting as central atoms. Examples Give the chemical structures and geometries of the following molecules. Label each as polar or nonpolar. PBr5 TeCl4 IOF2- Six-Coordinate Molecules Take on an Octahedral Geometry Unlike a trigonal bipyramid, the equatorial and axial positions in an octahedral are equivalent. When placing lone pairs in the structure, we must still maximize their distance. It is customary to first place lone pair in the axial positions. Lone Pairs Migrate As Far Away From One Another As Possible First electron pair is placed in an axial position If a second lone pair exists, it is placed the maximum distance (180o) from the 1st pair Total Electron Domains Domain Geometry Bonding Lone Pair Around Domains Domains Central Atom MOLECULAR GEOMETRY Octahedral B 6 0 B B B A B B Ex. SF6 6 Square pyramidal 5 1 •• B B B A B B Ex. BrF5 Total Electron Domains Domain Geometry Bonding Lone Pair Around Domains Domains Central Atom MOLECULAR GEOMETRY Square Planar •• 6 4 2 B B B A B •• Ex. XeF4 Examples • Give the chemical structures and geometries of the following molecules. Label each as polar or nonpolar. – SeF6 – ICl5 – XeF4