Slide 1
advertisement

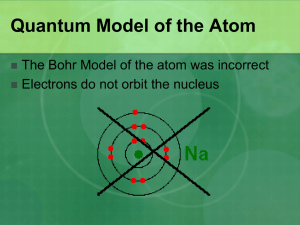
AP Chapter 6 Electronic Structure of Atoms HW: 5 7 29 35 63 67 69 71 73 75 6.1 – Wave Nature of Light -Electromagnetic Radiation = Emission and transmission of energy in the form of waves -examples: visible light, infrared, UV, X-Rays -Electromagnetic wave = Travels at the speed of light = 3.0x108 m/s in a vacuum Electromagnetic Spectrum = (display of electromagnetic radiation by wavelength) 6.1 – Wave Nature of Light -Wavelength = l = Distance between identical points on successive waves. Unit = Angstrom (A), nm, mm -Frequency = n = # of waves passing a point in a given unit of time (typically 1 second). Unit = Hz = 1/s -Speed of the wave = c = ln -Amplitude = Height -Node = Amplitude = 0 A high frequency wave must have a short wavelength A low frequency wave must have a long wavelength 6.1 – Wave Nature of Light Calculate: The yellow light given off by a sodium vapor lamp used for public lighting has a wavelength of 589 nm. What is the frequency of this radiation? (answer = 5.09 x 1014 s-1) 6.2 – Quantized Energy and Photons • Wave nature alone cannot explain all behaviors of light – Emission of light from hot objects (blackbody radiation) – Emission of electrons from metal surfaces on which light shines (photoelectric effect) – Emission of light from electronically excited gas atoms (emission spectra) 6.2 – Quantized Energy and Photons • He concluded that energy of a single quantum is equal to a constant times the frequency of the radiation: E = hn where h is Planck’s constant, 6.63 10−34 Js. • Max Planck explained it by assuming that energy comes in “chunks” called quanta. • Quantum = The smallest quantity of energy that can be emitted or absorbed • Quantum Theory – Atoms and molecules emit and absorb energy in discrete quantities only • Photon = A quantum of light energy • Therefore, if one knows the wavelength of light, one can calculate the energy in one photon, or packet, of that light: c = ln E = hn 6.2 – Quantized Energy and Photons a) A laser emits light with a frequency of 4.69 x 1014 s-1. What is the energy of one photon of the radiation from this laser? b) If the laser emits a pulse of energy containing 5.0 x 1017 photons, what is the total energy of that pulse? c) If the laser emits 1.3 x 10-2 J of energy during a pulse, how many photons are emitted during the pulse? Answers: a) 3.11 x 10-19 J b) 0.16 J c) 4.2 x 1016 photons 6.3 – Line Spectra and the Bohr Model Emission Spectrum = Spectrum of radiation emitted by a substance/energy source – can be continuous or line spectrum depending on the substance (continuous spectrum) When observing the emission spectra of atoms/molecules, only a line spectrum of discrete wavelengths is observed. (line spectrum) 6.3 – Line Spectra and the Bohr Model The energy absorbed or emitted from the process of electron promotion or demotion can be calculated by the equation: E = -RH ( 1 nf2 - 1 ni 2 ) where RH is the Rydberg constant, 2.18 10−18 J, and ni and nf are the energy levels of the electron -If nf is smaller than ni, then the e- moves closer to the nucleus and E is negative -If nf is larger than ni, then the e- moves farther from the nucleus and E is positive -Each line on the line spectrum of Hydrogen can be calculated using this equation. 6.3 – Line Spectra and the Bohr Model Ground State = Lowest energy state for the electron Excited State = A higher energy state 6.3 – Bohr’s Model of the Hydrogen Atom • Niels Bohr adopted Planck’s assumption and explained these phenomena in this way: 1. Electrons in an atom can only occupy certain orbits (corresponding to certain energies). 2. Electrons in permitted orbits have specific, “allowed” energies; these energies will not be radiated from the atom. 3. Energy is only absorbed or emitted in such a way as to move an electron from one “allowed” energy state to another; the energy is defined by E = hn Quantum Mechanics Preview: 6.4 - The Wave Behavior of Matter • Louis de Broglie posited that if light can have material properties, matter (electrons in atoms) should exhibit wave properties. • He demonstrated that the relationship between mass and wavelength was: h l = mn This equation relates the wave (l) and particle (m) natures 6.4 – The Wave Behavior of Matter • Heisenberg Uncertainty Principle: Heisenberg showed that the more precisely the momentum of a particle is known, the less precisely its position known It is impossible to know both the momentum and the position of an electron 6.5 - Quantum Mechanics • Erwin Schrödinger developed a mathematical treatment (Schrodinger Wave Equation) into which both the wave and particle nature of matter could be incorporated. • It is known as quantum mechanics. 6.5 -Quantum Mechanics • Uses advanced calculus • The wave equation is designated with a lower case Greek psi (). • The square of the wave equation, 2, gives a probability density map of where an electron has a certain statistical likelihood of being at any given instant in time = electron density Orbitals and Quantum Numbers • Solving the wave equation gives a set of wave functions, or orbitals, and their corresponding energies. • Each orbital describes a spatial distribution of electron density. • An electron is described by a set of four quantum numbers. Principal Quantum Number, n • The principal quantum number, n, describes the energy level on which the orbital resides. • It is a measure of the distance from the nucleus. • The values of n are integers ≥ 0. • Now 1-7 Angular Momentum Quantum Number, l • This quantum number defines the shape of the orbital. • Allowed values of l are integers ranging from 0 to (n − 1). • We use letter designations to communicate the different values of l and, therefore, the shapes and types of orbitals. Angular Momentum Quantum Number, l Value of l 0 1 2 3 Type of orbital s p d f Magnetic Quantum Number, m l • Describes the three-dimensional orientation of the orbital. • Values are integers ranging from - l to l : −l ≤ ml ≤ l • Therefore, on any given energy level, there can be up to – – – – s = 0 = one s orbital p = -1, 0, 1 = three p orbitals d= -2,-1,0,1,2 = five d orbitals, f=-3,-2,-1,0,1,2,3 = seven f orbitals Magnetic Quantum Number, m l • Orbitals with the same value of n form a shell. – Ex – The n=3 shell has an s orbital, three p orbitals and five d orbitals • The set of orbitals with the same shape within a shell form a subshells. – Ex = there are three orbitals in a p subshell) Spin Quantum Number • Each e- in one orbital must have opposite spins • Symbol – ms • +½,-½ – Two “allowed” values and corresponds to direction of spin 6.6 – Representation of Orbitals The s Orbital • Value of l = 0. • Spherical in shape. • Radius of sphere increases with increasing value of n. • Radius of sphere increases with increasing energy of the electron(s). p Orbitals • Value of l = 1. • Have two lobes with a node (no probability of finding electron) between them. d Orbitals • Value of l is 2. • Four of the five orbitals have 4 lobes; the other resembles a p orbital with a doughnut around the center. f Orbitals Higher than f not currently needed… AP EXAM QUESTIONS: List the four quantum numbers for the valence electrons in magnesium. List in order – n, l, m l , ms Valence electrons: 3s2 1 Electron 1 = 3,0,0, 2 Electron 2 = 3,0,0,- 1 2 AP EXAM QUESTIONS: List the four quantum numbers for the valence electrons in Nitrogen. Energies of Orbitals • For a one-electron hydrogen atom, orbitals on the same energy level have the same energy. • That is, they are degenerate. 6.7 – Many Electron Atoms • As the number of electrons increases, though, so does the repulsion between them. • Therefore, in manyelectron atoms, orbitals on the same energy level (principle quantum number) are no longer degenerate. • This is the order we use from the periodic table to fill orbitals = Aufbau Principle = electrons fill from low to high energy Pauli Exclusion Principle • No two electrons in the same atom can have exactly the same energy. • Also means that no two electrons in the same atom can have identical sets of quantum numbers. 6.8 - Electron Configurations • Shows the distribution of all electrons in an atom • Consist of – Number denoting the energy level Electron Configurations • Distribution of all electrons in an atom • Consist of – Number denoting the energy level – Letter denoting the type of orbital Electron Configurations • Distribution of all electrons in an atom. • Consist of – Number denoting the energy level. – Letter denoting the type of orbital. – Superscript denoting the number of electrons in those orbitals. Practice: N, Zr, Bi Orbital Diagrams • Each box represents one orbital. • Half-arrows represent the electrons. • The direction of the arrow represents the spin of the electron. Practice: B, Si Hund’s Rule “For degenerate orbitals, the lowest energy is attained when the number of electrons with the same spin is maximized.” (In a p, d or f subshell, fill each orbital with ONE electron before pairing any) • Diagmagnetism = Repelled by a magnet = Has all paired electrons with opposite spins • Paramagnetism = Attracted to a magnet = has at least one unpaired electron • Shielding = Inner electrons block outer electrons from the electrostatic force of the nucleus • Valence electrons = Outer-shell electrons involved in bonding Condensed Electron Configurations • Begin at the NOBLE GAS (Has full valence shell) before the element. Write that symbol in [brackets] • Continue on with the rest of the configuration Practice: Na, As, Ag, At Periodic Table • Different blocks on the periodic table, then correspond to different types of orbitals. Some Anomalies Some irregularities occur when there are enough electrons to half-fill s and d orbitals on a given row. Some Anomalies Group 6: Ex - Chromium is [Ar] 4s1 3d5 rather than the expected [Ar] 4s2 3d4. Group 11: Ex - Copper is [Ar] 4s1 3d10 rather than the expected [Ar] 4s2 3d9. Some Anomalies • This occurs because the 4s and 3d orbitals are very close in energy and a half-filled d is more stable than “missing” 1 e-. • These anomalies occur in f-block atoms, as well (Sm and Pu and Tm and Md)