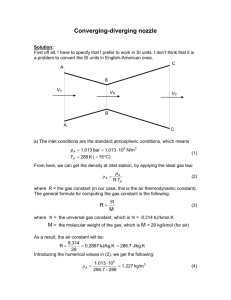
Why Arbitrary Component Reference States

Arbitrary Component Reference States
for the Heat-of-Reaction Method without Reaction
Reference State Misconceptions
“It doesn’t matter what temperature and pressure you select for a component reference state, because the enthalpy references will end up canceling out in the energy balance equation.
”
“Which component reference state is selected does not matter because the change in enthalpy between two states will always be the same no matter what the reference state.
”
Because the first statement is incorrect, it is a misconception. Although the second statement is correct, it does not explain why a component reference state can be arbitrarily defined. It too is a misconception about what role reference enthalpies play in the energy balance equation.
Why do these misconceptions exist? We will not try to answer this question. Instead, we will look at why the misconceptions are wrong by examining the process of heating pure water from
20ºC to 80ºC at 1 atm and for a flow rate of 100 kg/h. This process represents a sensible energy change in the liquid phase.
5.i
6.i
Process Diagram
Q
T
1
T
2
P
1 1 2
P
2 m
2 m
1 heater w
1, WA w
2, WA
Incorrect Interpretation
Eqn #
1.i
2.i
Misleading Derivation m
1
m
2
0 m
1, WA
m
2, WA
0
3.i m H
1
m H
2
Q
4.i
Factoring out the total flow rate in Eq. 3.i and rearranging gives:
Q
(
ˆ
2
ˆ
1
)
Specific enthalpies for Streams 1 and 2 are determined by:
H
1
2
WA
WA
Assumptions
1.
continuous process
2.
steady state
3.
no chemical reaction
4.
neglect KE and PE changes
5.
no shaft work
6.
pure water in the streams
0
ˆ
WA
1
,
1
T
WA
, P
WA
ˆ
WA
2
,
2
T
WA
, P
WA
Comments the total material balance the component water balance the energy balance because m
1
m
2 where
H
WA
is the enthalpy for pure water at an arbitrarily
-selected reference state of
T
WA and P
WA
.
7.i
Substituting these two enthalpies into Eq. 4.i gives:
Q
m
1
ˆ
WA
ˆ
WA
2
,
2
WA
ˆ
WA
1
,
1
the
notation has been dropped for clarity purposes
Simplifying Eq. 7.i gives:
8.i Q
m
1
ˆ
WA
2
,
2
ˆ
WA
1
,
1
Page 1 of 2
Arbitrary Component Reference States
for the Heat-of-Reaction Method without Reaction
This misleading derivation leads to the misconception that the reference enthalpies cancel and because of that fact only the change in enthalpy is what is important. If you apply the logic of this derivation to the case of mixing two liquid streams of water to form a third liquid stream, you would discover that this logic does not work, because the reference enthalpies cannot be cancelled.
Correct Interpretation
Eqn # Comments
1.c
2.c m
Proper Derivation m
1
m
2
0
1, WA
m
2, WA
0 the total material balance the component water balance
3.c
4.c
5.c m H
1
H
1
Q
m H
2
m H
2 w
1 , W A
H
W A
Q
m H
1
0 mix for a mixture the energy balance rearranged energy balance
ˆ
1
hmix
T
1
, P
1
, w
1
Multiplying Eq. 5.c with
1
,
gives:
6.c
7.c
8.c
9.c
1
H
1
1 , W A W A no mixing effect since pure m
1 1
hmix
T
1
, P
1
,
1 , j
' s
Specific enthalpy of pure water in Eq. 6.c is calculated by:
W A
W A
T
W A
, P
W A
, Ph
W A
W A
T
1
,
term is set arbitrarily
to zero for pure WA
P
1
, Ph
1
T
W A
, P
W A
, Ph
W A
units of kJ/kg
term is the enthalpy change to go from
pure WA reference state to mixture state
Simplifying the notation in Eq. 7.c gives:
W A
W A
H
W A
T
1
, P
1
specific enthalpy of pure WA
Substituting Eq. 8.c into Eq. 6.c gives:
1
ˆ
1
1 , W A
W A
W A
T
1
, P
1
, kJ / h
1 1
hmix
T
1
, P
1
,
1 , j
' s
10.c
Applying the same procedure used in Eqs. 5.c thru 9.c, the energy content of Stream 2 is:
2 2
2 , W A
W A
W A
T
2
, P
2
, kJ / h
2 2
hmix
T
2
, P
2
, m
2 , j
' s
where
W A in Eqs. 9.c and 10.c is at the reference state of
T
W A and
P
W A for pure water.
11.c
Substituting Eqs. 9.c and 10.c into the energy balance of Eq. 4.c and factoring the reference enthalpy terms gives:
Q
m
2, WA m
2, WA
ˆ
WA
2
,
2
m
1, WA
ˆ
WA
m
1, WA
ˆ
WA
1
,
1
energy balance equation component reference state
This proper derivation leads to the correct generalization that a component reference enthalpy is always multiplied by zero (i.e., its component balance). Because of this fact, you can arbitrarily pick a reference temperature ( T j
) and pressure ( P j
) for a component and assign any value to its reference enthalpy (
j
).
For convenience, we usually assign zero to the reference enthalpy. Note that Eq. 11.c in the “Correct
Interpretation” is equivalent to Eq. 8.i under the “Incorrect Interpretation”, since
m
2, WA
m
1, WA
0 for the water component balance, and m
2, WA
m
1, WA
m
2
m
1
for heating a process stream of pure water.
Page 2 of 2