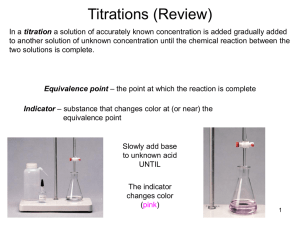
Acid-Base III
advertisement

Be able to write the chemical equation for a chemical species acting as an acid: HX(aq) H+(aq) + X-(aq) Example Write the equation showing the bicarbonate ion acting as an acid. HCO3-(aq) H+(aq) + CO32-(aq) Be able to write the chemical equation for a chemical species acting as a base: X-(aq) + H2O(l) OH-(aq) + HX(aq) Example Write the equation showing the bicarbonate ion acting as a base. HCO3-(aq) + H2O(l) OH-(aq) + H2CO3(aq) The Common Ion Effect What is the pH of a solution made by adding 0.30 mol of acetic acid to enough water to make 250 mL of solution? Ka is 1.8 x 10-5 at 25°C. CH3COOH(aq) initial change 0.30/0.250 M -xM equilibrium 1.2 - x M Ka = [CH3COO-][H+] = x2 [CH3COOH] (1.2 - x) CH3COO-(aq) + H+(aq) 0M 0M +x M +x M xM xM = 1.8 x 10-5 assume x<<1.2 x2 = 2.16 x 10-5 x = 0.0046 M (<<1.2) pH = -log(0.0046) = 2.33 verify your assumption The Common Ion Effect What is the pH of a solution made by adding 0.30 mol of acetic acid and 0.30 mol of sodium acetate to enough water to make 250 mL of solution? CH3COOH initial CH3COO-(aq) + H+(aq) 0.30/0.250 M change -xM equilibrium 1.2 - x M Ka = [CH3COO-][H+] = (1.2 + x)x [CH3COOH] (1.2 - x) x = 1.8 x 10-5 0.30/0.250 M 0M +x M +x M 1.2 + x M xM = 1.8 x 10-5 (assume x<<1.2) verify your assumption M (x<<1.2) pH = -log(1.8 x 10-5) = 4.74 The Common Ion Effect Adding acetate ion shifted the equilibrium to the left, decreasing [H+] and increasing pH. CH3COOH CH3COO-(aq) + H+(aq) adding C2H3O2- Common Ion Effect The extent of ionization of any weak electrolyte is decreased by adding to the solution a strong electrolyte that has an ion in common with the weak electrolyte. The Common Ion Effect a) Find the solubility of calcium phosphate in water at 25°C. b) Find the solubility of calcium phosphate in 0.100M sodium phosphate at 25°C. The Common Ion Effect a) Find the solubility of calcium phosphate in water at 25°C. What information do you need? formula for calcium phosphate source: your head (I hope) the equation for the equilibrium source: your head the equilibrium constant source: Appendix D The Common Ion Effect a) Find the solubility of calcium phosphate in water at 25°C. Ca3(PO4)2(s) 3Ca2+(aq) + 2PO43-(aq) Ksp = 2.0 x 10-29 I present C lose x mol E present 2.0 x 10-29 = 108x5 +3x M +2x M 3x M 2x M x = 7.1 x 10-7 M The Common Ion Effect b) Find the solubility of calcium phosphate in 0.100M sodium phosphate at 25°C. Ca3(PO4)2(s) 3Ca2+(aq) + 2PO43-(aq) Ksp = 2.0 x 10-29 I present C lose x mol E present 0.100 M +3x M 3x M +2xM (0.100+2x) M Assume 2x << .100 2.0 x 10-29 = .27x3 x = 4.2 x 10-10 M Check: 8.4 x 10-10 << .1 (100,000,000 times smaller) The Common Ion Effect a) Find the solubility of calcium phosphate in water at 25°C. 7.1 x 10-7 M b) Find the solubility of calcium phosphate in 0.100M sodium phosphate at 25°C. 4.2 x 10-10 M The presence of phosphate from sodium phosphate decreased the solubility of the calcium phosphate by a factor of a 1000. Buffered Solutions What is the pH of our 1.2M acetic acid solution if 10.0 mL of 1.0 M NaOH is added to it? CH3COOH CH3COO-(aq) + H+(aq) If NaOH is added to the solution, it reacts with some of the acetic acid: CH3COOH(aq) + NaOH(aq) CH3COONa(aq) + H2O(l) This has the effect of decreasing the acetic acid concentration and increasing the acetate ion concentration. PLUS, addition of the NaOH changes the volume of the system. Buffered Solutions What is the pH of our 1.2M acetic acid solution if 10.0 mL of 1.0 M NaOH is added to it? CH3COOH CH3COO-(aq) + H+(aq) If NaOH is added to the solution, it reacts with some of the acetic acid and produces acetate ion: CH3COOH(aq) + NaOH(aq) CH3COONa(aq) + H2O(l) This is a stoichiometry problem. Buffered Solutions What is the pH of our 1.2M acetic acid solution if 10.0 mL of 1.0 M NaOH is added to it? CH3COOH initial: change: CH3COO-(aq) (0.30 - 0.010)/0.260 M 0.010/0.260 M + H+(aq) 0M -xM +xM +x M equilibrium: 1.115 - x M 0.0385 + x M xM Ka = [CH3COO-][H+] = (.0385 + x)x [CH3COOH] (1.115 - x) = 1.8 x 10-5 (assume x<<0.0385) x2 + .0385x - 2.01x10-5 = 0 x = 5.2 x 10-4 M (x<<0.0385) pH = -log(5.2 x 10-4) = 3.29 verify your assumption Buffered Solutions What is the pH of our acetic acid/sodium acetate solution if 10.0 mL of 1.0 M NaOH is added to it? CH3COOH initial: CH3COO-(aq) + H+(aq) (0.30-.010)/0.260 M (0.30+.010)/0.260 M 0 M change: -xM +x M +x M equilibrium: 1.115 - x M 1.192 + x M xM Ka = [CH3COO-][H+] = (1.192 + x)x = 1.8 x 10-5 [CH3COOH] (1.115 - x) (assume x<<1.115, 1.192) x = 1.7 x 10-5 M (x<<1.115 and 1.192) pH = -log(1.7 x 10-5) = 4.77 verify your assumption Buffered Solutions HAc is short for acetic acid. pH pH after addition of 10.0 mL of 1.0 M NaOH pH after addition of 10.0 mL of 1.0 M HCl 250 mL of 1.2 M HAc 250 mL of 1.2 M HAc/Acbuffer soln 2.33 3.29 1.47 4.74 4.77 4.71 7.00 12.59 1.41 Ac- is short for acetate. 250 mL of DI water Buffered Solutions pH pH after addition of 10 mL 1.0 M NaOH pH after addition of 10 mL 1.0 M HCl 250 mL of 1.2 M HAc 2.33 3.29 1.47 4.74 4.77 4.71 7.00 12.59 1.41 250 mL of 1.2 M HAc/Ac 250 mL of DI water • The pH of the HAc solution ranged 1.47 - 3.20 with the addition of 10.0 mL of the acid or base. Buffered Solutions pH pH after addition of 10 mL 1.0 M NaOH pH after addition of 10 mL 1.0 M HCl 250 mL of 1.2 M HAc 2.33 3.29 1.47 4.74 4.77 4.71 7.00 12.59 1.41 250 mL of 1.2 M HAc/Ac 250 mL of DI water • When the same amount of acid or base was added to the HAc/Ac solution, its pH ranged 4.71 - 4.77. The addition of the acetate ion buffers the solution against changes in pH. Buffered Solutions • contain a weak conjugate acid-base pair such as HAc/Ac-, NH3/NH4+, H2CO3/HCO3-, HCN/CN-. • resist changes in pH because they contain an acid to react with OH- and a base to react with H+. • have the ability to resist changes in pH, known as their buffer capacity, which depends on the concentrations of the members of the pair. The pH of a buffer solution may be determined by the Henderson-Hasselbalch equation. Henderson-Hasselbalch Equation For the general equilibrium HX(aq) H+(aq) + X-(aq) the pH is given by [𝒃𝒂𝒔𝒆] 𝒑𝑯 = 𝒑𝑲𝒂 + 𝒍𝒐𝒈( ) [𝒂𝒄𝒊𝒅] Henderson-Hasselbalch equation Henderson-Hasselbalch Equation • Let’s recalculate the pH of our 1.2 M HAc/Ac solution. pH = pKa + log ([base]/[acid]) Ka = 1.8 x 10-5, so pKa = 4.74 pH = 4.74 + log(1.2M/1.2M) = 4.74 • After the addition of 10.0 mL 1.0 M NaOH pH = 4.74 + log(1.192M/1.115M) = 4.74 + 0.03 = 4.77 Buffered Solutions You have been asked to prepare 500.0 mL of a buffer with the pH of blood, 7.40. What chemicals and amounts will you use? Buffers most effectively resist a change in pH in either direction if they contain equal concentrations of both members of the acidbase pair. Find a weak acid with a Ka ≈ 10-7 (so the pKa will be 7). Ka3(citric acid) = 4.0 x 10-7 at 25°C. pKa = 6.40 7.40 = 6.40 + log [base]/[acid] [base]/[acid] = 10 Buffered Solutions You have been asked to prepare 500.0 mL of a buffer with the pH of blood, 7.40. What chemicals and amounts will you use? We chose citric acid (abbreviated H3Cit) and found that we need a base-to-acid ratio of 10. For Ka3, the base is Cit3- and the acid is HCit2-. HCit2-(aq) Ka3 = 4.0 x 10-7 at 25°C pH = pKa3 + log [Cit3-] [HCit2-] Cit3-(aq) + H+(aq) Buffered Solutions Solution #1: Put 1.0 mol of Na3Cit and 0.10 mol Na2HCit in a 500-mL volumetric flask and dilute to the mark with DI water. Solution #2: Put 0.010 mol of Na3Cit and 0.0010 mol Na2HCit in a 500-mL volumetric flask and dilute to the mark with DI water. Both solutions will produce the correct pH…what is different about them? Their BUFFERING CAPACITY. Solution #2 has smaller concentrations of the acid-base pair. Titration of a Strong Acid with a Strong Base If we neutralize an acid incrementally and monitor the pH of the solution as we add the base, the resulting data would give us a titration curve, a plot of pH versus volume of titrant added. Titration of 0.100 M HCl 14.00 12.00 10.00 pH 8.00 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Strong Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HCl with 0.100 M NaOH, the titration curve would be a plot of pH versus volume of NaOH added. Titration of 0.100 M HCl 14.00 12.00 10.00 pH 8.00 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Strong Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HCl with 0.100 M NaOH, here is some useful information: • 50.0 mL of 0.100 M HCl contain 5.00 mmol H+ • 1.0 mL of 0.100 M NaOH contains 0.10 mmol of OH𝒎𝒎𝒐𝒍 𝒎𝑳 = 𝒎𝒐𝒍 𝑳 = molarity (M) Titration of a Strong Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HCl with 0.100 M NaOH, the [H+] before the equivalence point is the concentration of unreacted HCl. After 25.0 mL of NaOH have been added, the total volume is (50.0 + 25.0) mL, and [H+] =(5.00mmol HCl - 2.50mmol NaOH)/75 mL = 0.033 M pH = 1.48 Titration of a Strong Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HCl with 0.100 M NaOH, the [OH-] after the equivalence point is the concentration of excess NaOH. After 75.0 mL of NaOH have been added, the total volume is (50.0 + 75.0) mL, and [OH-] = (7.50 mmol NaOH - 5.00 mmol HCl)/125 mL = 0.0200 M pOH = 1.70 pH = 14.00- 1.70 = 12.30 Titration of a Strong Acid with a Strong Base Titration of 0.100 M HCl 14.00 12.00 The initial pH depends solely on the concentration of the acid. Here it is 1.00. 10.00 8.00 pH The pH at the equivalence point is 7. This is true for the titration of any strong acid by any strong base…and vice versa. 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Weak Acid with a Strong Base For the neutralization of 50.0 mL of 0.10 M HAc with 0.100 M NaOH, the [H+] before the equivalence point is calculated from the equilibrium expression using the concentration of unreacted HAc. After 25.0 mL of NaOH have been added, the total volume is (50.0 + 25.0) mL, and HAc (5.00-2.50)/75.0 M Ac-(aq) + H+(aq) 2.50/75.0 M We can use the Henderson-Hasselbalch equation: Ka = 1.8 x 10-5 pKa = 4.74 [Ac-] = [HAc] = 2.50/75.0 M [base]/[acid] = 1.00 pH = 4.74 + log 1.00 = 4.74 Titration of a Weak Acid with a Strong Base Titration of 0.100 M Acetic Acid 14.00 The initial pH depends on the concentration of the weak acid and its Ka. 12.00 10.00 pH 8.00 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Weak Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HAc with 0.100 M NaOH, the [H+] at the equivalence point is calculated from the equilibrium expression for the base. After 50.0 mL of NaOH have been added, the total volume is (50.0 + 50.0) mL, and all of the HAc has been changed to Ac-. Ac-(aq) initial: change: equilibrium: 5.00/100.0 M -xM 0.0500 - x M + H2O(l) HAc(aq) + OH-(aq) 0M 0M +xM +xM xM xM Kb = Kw/Ka = 1.0 x 10-14/(1.8 x 10-5) = 5.6 x 10-10 [OH-][HAc] = 5.6 x 10-10 ≈ x2 x = [OH-] = 5.3 x 10-6 [Ac-] 0.0500 pOH = 5.28 pH = 14.00- 5.28 = 8.72 Titration of a Weak Acid with a Strong Base Titration of 0.100 M Acetic Acid 14.00 12.00 10.00 pH 8.00 6.00 4.00 The pH at the equivalence point is 8.72. 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Weak Acid with a Strong Base For the neutralization of 50.0 mL of 0.100 M HAc with 0.100 M NaOH, the [OH-] after the equivalence point is the concentration of excess NaOH. This is exactly the same as the postequivalence section of the strong acid-strong base titration curve. After 75.0 mL of NaOH have been added, the total volume is (50.0 + 75.0) mL, and [OH-] = (7.50 mmol NaOH - 5.00 mmol HCl)/125 mL = 0.0200 M pOH = 1.70 pH = 14.00- 1.70 = 12.30 Titration of a Weak Acid with a Strong Base Titration of 0.100 M Acetic Acid 14.00 12.00 This part of the curve is identical to that of the HCl/NaOH curve, since in both cases the NaOH volume added is the same. 10.00 pH 8.00 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Weak Base with a Strong Acid The titration curve for a weak base with a strong acid is calculated similarly to that of the weak acid/strong base. You must be careful to write the equilibrium expression correctly and use the correct Kb. For the titration of aqueous ammonia, use NH3(aq) + H2O(l) Kb = 1.8 x 10-5 at 25°C NH4+(aq) + OH-(aq) Titration of a Weak Base with a Strong Acid Titration of 0.100 M Ammonium Hydroxide 12.00 10.00 pH 8.00 6.00 4.00 2.00 0.00 0 20 40 60 mL 0.100 M HCl added 80 100 Region of Maximum Buffer Capacity [𝒃𝒂𝒔𝒆] 𝒑𝑯 = 𝒑𝑲𝒂 + 𝒍𝒐𝒈( ) [𝒂𝒄𝒊𝒅] Buffering is best when [base] = [acid]. But when [base] = [acid], pH = pKa This occurs when the volume of NaOH added is ½ the NaOH volume needed to reach the equivalence point. Region of Maximum Buffer Capacity Titration of 0.100 M Acetic Acid 14.00 12.00 10.00 pH 8.00 6.00 4.00 pH = pKa = 4.72. Maximum buffering capacity is here. 2.00 0.00 0 20 40 60 mL 0.100 M NaOH added 80 100 Titration of a Polyprotic Acid with a Strong Base 13.00 2nd equivalence point pH = 9.9 12.00 11.00 H2A is completely neutralized at 2nd eq pt. 10.00 9.00 8.00 1st equivalence point pH = 4.5 7.00 pH 6.00 5.00 HA- and A2- 4.00 3.00 2.00 1.00 H2A and HA- 0.00 0 10 20 30 40 50 60 volume of NaOH added 70 80 90 100 Titration of a Polyprotic Acid with a Strong Base 13.00 halfway between 40 and 80 mL 12.00 11.00 10.00 9.00 pKa2= 7.2 8.00 7.00 pH halfway between 0 and 40 mL 6.00 5.00 HA- and A2- 4.00 3.00 pKa1= 2.0 2.00 1.00 H2A and HA- 0.00 0 10 20 30 40 50 60 volume of NaOH added 70 80 90 100 Not All Equivalence Points are Obvious



![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)