H +1 - Milwaukie High
advertisement

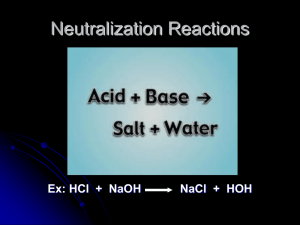
Acids/Bases Notes One Unit Eleven Pages 541-549 Properties of Acids Properties of Bases Structure of Bases Neutralization Reactions Lab Acid/Base Properties Next Class Part I Effect of acids and base on indicators • 1. Stand 3 clean, dry test tubes in the test tube rack. Add about 2 mL of 6M HCl to one of the test tubes, 2 mL of 6M HC2H3O2 to a second tube, and 2 mL of 0.5 M NaOH to the third tube. CAUTION: Handle these chemicals with care. Test each solution first with red litmus paper and then with blue litmus paper. • 2. Test each solution with pH paper. • 3. Add 1 drop of phenolphthalein to each solution. Discard the solutions as instructed. Clean and rinse the test tubes. Part IV Neutralization • 1. Using a clean dropper pipette, add 10 drops of 1.0M HCl and 1 drop of phenolphthalein to a test tube. Test with pH paper. • 2. Using a second dropper pipette, add 0.5M NaOH drop-by-drop. After the addition of each drop, swirl the test tube gently mix the contents. Count the total number of drops of NaOH needed to cause a color change. Once a color change is observed, test the mixture with pH paper. Number of drops of 0.5M NaOH to neutralize 10 drops of 1.0M HCl _________. pH of neutral solution:_________ Lime Water Test For CO2 Zinc with hydrochloric acid Metals With Acid Questions on Lab • 5. What type of reaction occurs between a metal and an acid? Write a general equation for this type of reaction. • Single • M+ A------->H2+ salt • 6. Explain the difference in reaction rates of a given metal with two different acids. • HCl is a strong acid. It makes more H3O+1. More H3O+1 means faster reaction. • 7. Write a balanced equation for the reaction between CO2 gas and lime water, Ca(OH)2. What is the name of the milky precipitate that forms? • Ca(OH) 2 + CO2 ------>CaCO3+ H2O • 8. Explain the difference in the volumes (number of drops) of HCl and NaOH required to produce a neutral solution in Part 4 of this experiment. • 10 drops to 20 drops means the base is half the concentration. Notes One Unit Eleven Properties of Acids Properties of Bases Structure of Bases Neutralization Reactions Properties of Acids Sour taste. warhead React with “active” metals produce H2. Al, Zn, and Fe React with carbonates, producing CO2 and H2O. Marble, baking soda, chalk, limestone. Change color of vegetable dyes. cabbage juice / On the top cabbage juice / baking soda (left) cabbage juice / vinegar (right). Acids acid - proton donor (H+1) strong vs. weak acids strong - lots of (H+ ) weak - very little (H+ ) HCl is a strong acid Muriatic acid is hydrochloric acid. Making Bleach. Making PVC pipe. Making Table Salt. Human stomach acid. Cleaning steel. Neutralize bases in chemical plants. Chrome tanning leather. HNO3 is a strong acid Explosives Fertilizers etc. Nitrate salts To make H2SO4 Etching copper, brass, bronze Dyes, perfumes Purification of Ag, Au, Pt Properties of Bases Also known as alkali.( Li, Na, K, Rb, Cs, Fr) Taste bitter. caffeine often poisonous. Solutions feel slippery. Change color of vegetable dyes. Different color than acid. Red litmus turns blue. React with acids to form salt and water(Neutralization). Acid + base salt +water Common Base Features Most ionic bases contain OH-1 ions. NaOH (drain cleaner) Some contain CO32- ions. CaCO3(in Tums) NaHCO3 (baking soda) Molecular bases contain structures that react with H+. Mostly amine groups(-NH2). Neutralization Reactions acid + base salt + water Double-displacement Some make CO2 and H2O Acid Reactions Acid+carbonateSalt +Water +Carbon Dioxide Acid+Base Salt +Water Acid+Metal Salt +Hydrogen Acid and Carbonate Acid+ CarbonateSalt+ Water+Carbon Dioxide 2 HNO3+1 Na2CO3 2 NaNO3 +1 H2O + 1CO2 H+1 NO3-1 Na+1 CO3-2 Salt ? =Sodium Nitrate (Na+1)_( 1 NO -1 )_ 1 3 NaNO3 Acid and Base Water Acid+ Base Salt + 1 H2SO4+ 2 KOH 1 K2SO4 + 2 H2O H+1 SO4-2 K+1 OH-1 Salt ? =Potassium Sulfate ( K+1 )_( 2 SO -2 )_ 1 4 K2SO4 Acid and Metal Salt + Hydrogen Acid+ Metal 1 H2SO4 + 1 Mg(s) 1MgSO4 + 1 H2(g) H+1 SO4-2 Mg(s) Salt ? = Magnesium Sulfate 2 SO -2 )_ 2 ( Mg+2)_( 4 MgSO4 Titration Example One What is the molarity of a NaOH solution, if 12.01 mL is required to titrate 5.99 mL of 0.100 M HCl? 1. Balance the equation. 1NaOH(aq)+ 1 HCl(aq) 1 NaCl(aq) + 1 H2O(l) 2. Find moles used of known solution. MxV=n 0.100M x 0.00599L = 0.000599m HCl 3. Calculate moles used of unknown (titrant). 1 m NaOH 0.000599 m HCl x = 0.000599m NaOH 1 m HCl 4. Calculate the molar concentration of the titrant. n/V=M 0.000599m NaOH ÷0.01201L = 0.0499M NaOH Notes Two Unit Eleven -Text Pages 550-558 Self Ionization of Water Brönsted-Lowrey Acid-Base Theory Arrhenius Theory Pages 550-558 Self Ionization of Water • Water is and acid and a base at the same time…amphoteric • H2O(l) + H2O(l) H3O+ + OH• Mass Action Expression • Kw = [H3O+][OH-] • Or Kw = [H+][OH-] • Kw = 1.0 x 10-14 Brönsted-Lowrey Acid-Base Theory • • • • Acid - proton donor H+1 Base - proton acceptor Acid-Conjugate Base / Base-Conjugate Acid HCl(aq) + H2O(l) Cl−1(aq) + H3O+1(aq) A B CB CA • NaF(aq) + H2O(l) HF(aq) + NaOH(aq) B A CA CB • OH−1(aq) + H2O(l) H2O(l)+ OH−1(aq) B A B A CA CB • NH3(aq) + H2O(l) NH4+1(aq) + OH−1(aq) CA CB Arrhenius Theory • Bases form OH- ions in water. • Acids form H+ ions in water. Arrhenius theory • • • • HCl(aq) H+1(aq) + Cl−1(aq) HF(aq) H+1(aq) + F−1(aq) NaOH(aq) Na+1(aq) + OH−1(aq) NH3(aq) +H2O(aq) NH4+1(aq) + OH−1(aq) Polyprotic Acids • More than one proton to donate. • H2CO3(aq) H+1(aq) + HCO3-1(aq) • HCO3-1(aq) H+1(aq) + CO3-2(aq) • H2SO4 H+1(aq) + HSO4−1(aq) • HSO4−1 H+1(aq) + SO4−2(aq) Notes Three Unit Eleven Titration Pages 574-578 Titration • Titration is a technique to determine the concentration of an unknown solution. • Titrant (unknown solution) • Phenolphthalein identifies the Endpoint. Titration Endpoint • Add 10mL of HCl and three drops phenolphthalein to the flask. • Add about 8mL base, swirl and add the rest of the base using increasingly faster spins of the valve.(?????) Titration-Acid Volume • • • • • • • Acid Burette Initial Reading? 1.98mL Final Reading? 7.97mL Volume Used? 5.99mL Titration-Volume of Base Used. • • • • • • • Base Burette Initial Reading? 0.00mL Final Reading? 12.01mL Volume Used? 12.01mL Titration Example Two • Lactic acid Concentration of Sauerkraut • For Joe’s final in chemistry, he was asked to find the concentration of lactic acid in homemade sauerkraut. • He did not have any home made sauerkraut. • Therefore, Joe was left to make the home made sauerkraut. • He looked on line and found the following recipe. Clean and Quarter 35lb of Fresh Cabbage Shred the Cabbage into a Crock Add 3 Tbsp salt per 5 pounds of cabbage. Mix salt and cabbage. Pack the cabbage into a crock and weight it down. It should be fermented in one month. Titration Example Two What Is the molarity of a sauerkraut juice if 10.0 mL is titrated using 89.9 mL of 1.00 M NaOH? 1. Balance the equation. 1NaOH + 1 HC2H4OHCO2 1 NaC2H4OHCO2 + 1 H2O 2. Find moles used of known solution. MxV=n 1.00M x 0.0899L = 0.0899m NaOH 3. Calculate moles used of unknown (titrant). 1m HC2H4OHCO2 0.0899m NaOHx = 0.0899m HC2H4OHCO2 1 m NaOH 4. Calculate the Molar Concentration of theTitrant. n/V=M 0.0899m HC2H4OHCO2 ÷0.01201L = M M=0.0899M HC2H4OHCO2 Titration Example Three What is the volume of a 0.0622M Ba(OH)2 solution, if it is titrated using 43.8 mL of 0.1057 M HCl? 1. Balance the equation. 1Ba(OH)2(q) + 2 HCl(aq 1 BaCl2(aq)+ 2H2O(l) 2. Find moles used of known solution. MxV=n 0.1057M x0.0438L= 0.00463m HCl 3. Calculate moles used of unknown (titrant). 1 m Ba(OH)2 0.00463 m HCl x = 0.00232 m Ba(OH)2 2 m HCl 4. Calculate volume of titrant. n/M=V 0.00234m Ba(OH)2 ÷0.0622M = 0.0376M Ba(OH)2 Lab B Titration Results Trial #1 HCl NaOH Trial #2 HCl NaOH Trial #3 Trial #4 HCl NaOH HCl NaOH Intial reading 0.01 0.21 11.06 21.10 21.21 31.35 31.34 42.00 Final reading 11.05 11.27 21.00 31.54 31.33 42.07 38.95 49.99 Volume used 11.04 11.60 9.94 10.21 10.72 7.61 7.99 10.44 Table a Calculations • For each trial, calculate the molarity of the NaOH solution using the relationship • Conclusion and Question • 1. How reproducible were the results of your four trials? Conclusions continued • 2. Define these terms: standard solution; titration; endpoint. • Standard Solution: When the concentration of a solution is known to a high degree of accuracy and precision. • titration: When the concentration of an acid or base is determined by neutralizing it. • endpoint: The point where you actually stop a titration, usually because an indicator has changed color. This is different than the "equivalence point" because the indicator might not change colors at the exact instant that the solution is neutral. Notes Three Unit Eleven Weak Acid/Base pH Scale Calculating pH Pages 559-567 Which acid is stronger? 6M HCl zinc magnesium iron copper 6M HC2H3O2 Weak Acids and Bases • • • • • A weak acid little H+1 A weak base little OH-1 [H+] or [OH-] from a Keq. pH = -log[H+1] pH is a measure of the amount of hydrogen in a solution. It is based on the water. Stomach Vinegar pH Scale Acid Blood 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Neutral Acid Base 1M HCl Ammonia 1M NaOH Lemon Juice Milk Water pH +pOH =14 Cleaner pH of strong acid • Find the pH of a 0.15 M solution of Hydrochloric acid • pH = - log 0.15 • pH = - (- 0.82) • pH = 0.82 pH of a strong Base • What is the pH of the 0.0010 M NaOH solution? • pOH = - log (0.0010) • pOH = 3 pH +pOH =14 • pH = 14 – 3 = 11 Ionization Constants for Acids/Bases Calculating pH (pH=-log[H+1]) The Ka for nitrous acid is 4.5 x 10-4. Calculate the [H+1] and pH in 0.15 M nitrous acid solution. 1) Balanced Equation HNO2 NO2-1 + H+1 2) Mass Action Expression 3) What do we know? [HNO2 ] [NO2-1] Bef 0.15 0 Very small Δ +x -x At 0.15-x x [NO2-1] [H+1] Ka= [HNO ] 2 [H+1] 0 4.5 x 10-4 +x -1 1.5 x 10 x 4) Calculate the [H+1] and pH. [x][x] Ka= [0.15] = 4.5 x 10-4 pH=-log[H+1] pH=-log[0.0082M ]= 2.09 X=[0.0082M] = [H+1] Calculating Concentrations Using Ka The Ka for benzoic acid is 6.5 x 10-5. (a) Calculate the concentrations of C6H5COO-1 and H+ in a 0.10 M benzoic acid solution. (b) Calculate pH. 1) Balanced Equation C6H5COOH C6H5COO-1 + H+1 [C H COO-1][H+1] 6 5 Ka= [C6H5COOH] 2) Mass Action Expression 3) What do we know? [C6H5COOH] [C6H5COO-1] [H+1] Bef 0.10 0 small Δ Very -x +x At 0.10-x x 4) Calculate the [H+1] and pH. [x] [x] Ka= [0.10] 0 +x x 6.5 x 10-5 1.0 x 10-1 X=[0.0025M] = [H+1] = 6.5 x 10-5 pH=-log[0.0025M]= 2.60 End
![pH = - log [H + ]](http://s2.studylib.net/store/data/005622524_1-002df1ea50d2a849b15deb604928664e-300x300.png)