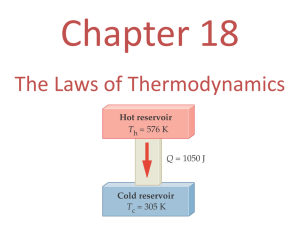
Lecture 01: Introduction to Thermodynamics and Units Review
advertisement

Thermodynamics EGR 334 Lecture 01: Introduction to Thermodynamics Today’s Objectives: • Distribute and understand syllabus • Take quick tour of thermodynamics topics covered in this course • Understand difference between system and control volume. • Review units of thermodynamics related quantities Reading Assignment: • Read Chap 1. Sections 1 - 9 Homework Assignment: From Chap 1: Problems 19, 37, 51, 59 Thermodynamics Definition -- Thermodynamics is the science of energy and entropy -- Thermodynamics: the branch of physics dealing with the relationships between heat, work, and forms of energy. From the Greek: - θερμη, therme, meaning “heat“ → Energy - δυναμις, dynamis, meaning “power“→ Transport To the Engineer…Thermodynamics means: - Understanding the 4 Laws of Thermodynamics - Learn to work in with four different temperature scales. - Learn to balance energy, heat, and work with respect to open and closed systems. - Learn about common thermodynamcis devices and applications and how the principles can be used to predict system performance and efficiencies. Thermo….a Quick Survey -- Properties of systems: Temperature, Pressure, Specific Volume, Phase, Quality, Density, Enthalpy, Entropy -- Processes and Cycles: State to state transitions. Carnot cycle, Rankine cycle, Otto cycle, Diesel cycle, Brayton cycle, Refrigeration cycle -- Work-Energy-Heat Balance: Applying the 1st Law of thermodynamics Thermo…A Quick Survey…continued -- Entropy Production and Accounting: Applying the 2nd Law of thermodynamics -- Thermodynamics devices: Turbines, Heat Exchangers, Condensers, Engines Pumps, Cooling Towers, Compressors, Diffusers -- Psychrometrics: HVAC, heating, ventilation, air conditioning, and humidity -- Combustion and Power Production: Chemical energy production and balances A basic concept: System • A System is whatever it is that we want to study. • In thermodynamics, the first step in defining any problem is to define exactly what is to be monitored, examined, measured, etc. Environment Interactions System Boundary System Definition Radiation Radiation System Definition Radiation Radiation System Definition Gas Q System Definition Gas Gas Q W Types of Systems • Isolated Systems – matter and energy may not cross the boundary. • Adiabatic Systems – heat may not cross the boundary. • Diathermic Systems - heat may cross boundary. • Closed Systems – matter may not cross the boundary. • Open Systems – heat, work, and matter may cross the boundary (more often called a Control Volume (CV)). Types of Systems • Isolated Systems – matter and energy may not cross the boundary. • Adiabatic Systems – heat must not cross the boundary. • Diathermic Systems - heat may cross boundary. • Closed Systems – matter may not cross the boundary. • Open Systems – heat, work, and matter may cross the boundary (more often called a Control Volume (CV)). Closed System • Matter (m) may not cross the boundary. • Heat (Q) and Work (W) may cross the boundary which change the energy (ΔE) of the system. Po, Vo, To Combustion CxHy+A O2 B CO2 + C H2O Pf, Vf, Tf Q ∆V →W Control Volume / Open System • Heat (Q), work (W), and matter (m) may cross the boundary Ga s Q System Properties • The State of a system is defined by its properties (T, V, P, E, ρ, v, u, h, s) • Extensive properties – Depends on mass • Intensive properties – Does not depend on mass. • Give some examples of Extensive Properties Intensive properties Process vs. Cycle. • A Process is when properties of the system undergo a change. A process moves from one “state” to another “state”. • If State i = State f then system is said to be Steady State. • If a Process undergo changes that eventually bring it back to the original state, these transitions together are called a Cycle. Process: Cycle: P P S1 S2 S2 S1 S4 S3 v v Unit Review: Weight and Mass Weight Mass U.S. Customary pound , lbf pound of mass, lbm or slug Metric Newton, N kilogram, kg W =m g where g = 9.81 m/s2 g = 32.2 ft/s2 1 N = 0.2248 lbf 1 lbf = 4.4482 N 1 kg = 2.205 lbm 1 lbm = 0.4536 kg 1 slug = 32.2 lbm = 71.0 kg Relationships: Conversions: Unit review: Density and Specific Volume Density ρ mass basis U.S. Customary [lbm / ft3 ] molar basis mass basis Specific Volume v or v [ ft3 /lbm] [ ft3 /mol] [kg/m3] [ m3/kg] Metric molar basis [m3/mol] Relationships m = mass V = Volume m V M = molecular weight n = number of moles n m M 1 M Unit review: Pressure Pressure = U.S. Customary Presure head [psi ] or [lbf /in2 ] [psf] or [lbf /ft2 ] [in of Hg ] [ in of H20 ] [ft of H20] Metric [N/m2] [ Pa] [bar] [mm of Hg ] [ mm of H20 ] [cm of H20] Other atm Relationships: 1 atm = 14.69 psi = 1.01325 bar = 100 324 Pa = 760 mm of Hg = 29.92 in of Hg = 33.96 ft of H2O Gage vs. Absolute pressure pabs = patm + pgage Vacuum vs. absolute pressure pabs = patm - pvac Unit review: Temperature Metric U.S. Relative Temperature Celcius [ oC ] Fahrenheit [ o F] Absolute Temperature Kelvin [K] Rankine [ oR ] Relationships K = oC + 273.15 oR = oF + 459.67 oC =( oF - 32)/1.8 oF= 1.8 oC + 32 oR = 1.8 K Example 1: An object whose mass is 35 lb is subjected to an applied upward force of 15 lbf. The only other force acting on the object is the force of gravity. Determine the net acceleration of the object in ft/s2 assuming the acceleration of gravity is constant (g = 32.2 ft/s2). Is the net acceleration up or down? FAF= 15 lbf mass= 35 lbm Fg= 15 lbf Example 2: A system consists of N2 in a piston-cylinder assembly, initially at P1 = 20 psi, and occupying a volume of 2.5 ft3. The N2 is compressed to P2 = 100 psi and a final volume of 1.5 ft3. During the process, the relationship between P and V is linear. Determine the P in psi at an intermediate state where the volume is 2.1 ft3 and sketch the process on a graph of P vs V. P1= 20 psi V1 =2.5 ft3 P2= 100 psi V2 =1.5 ft3 Example 3: A monometer is attached to a tank of gas in which the pressure is 104.0 kPa. The manometer liquid is mercury, with a density of 13.59 g/cm3. If g = 9.81 m/s2 and the atmospheric pressure is 101.33 kPa, calculate (a) The difference in mercury levels in the manometer, in cm. (b) The gage pressure of the gas in kPa and bar (c) The absolute pressure of the gas kPa, atm, and psi Example 1: An object whose mass is 35 lbm is subjected to an applied upward force of 15 lbf. The only other force acting on the object is the force of gravity. Determine the net acceleration of the object in ft/s2 assuming the acceleration of gravity is constant (g = 32.2 ft/s2). Is the net acceleration up or down? F ma FAF= 15 lbf m= 35 lbm Fg= ? FAF Fg ma FAF Fg FAF mg a m m 15 lb f 32.2 lbm ft a lb f 35 lbm a 18.4 ft s FAF g m s lbm ft 32.2 s Example 2: A system consists of N2 in a piston-cylinder assembly, initially at P1 = 20 psi, and occupying a volume of 2.5 ft3. The N2 is compressed to P2 = 100 psi and a final volume of 1.5 ft3. During the process, the relationship between P and V is linear. Determine the P in psi at an intermediate state where the volume is 2.1 ft3 and sketch the process on a graph of P vs V. Since the relationship is linear (y=mx+b) P2 P1 100 20 psi m 80 3 V V 1 . 5 2 . 5 ft 2 1 P P1 mV V1 P mV V1 P1 psi P 80 3 2.1 2.5 ft 3 20 psi 52 psi ft Example 3: A monometer is attached to a tank of gas in which the pressure is 104.0 kPa. The manometer liquid is mercury, with a density of 13.59 g/cm3. If g = 9.81 m/s2 and the atmospheric pressure is 101.33 kPa, calculate (a) The difference in mercury levels in the manometer, in cm. (b) The gage pressure of the gas in kPa and bar (c) The absolute pressure of the gas atm, and psi Solution: a) P Patm gL L 104.0 101.33 kPa P Patm g 103 N / m 2 103 g 1 kg m / s 2 (1m) 2 L (13.59b) g / cm3 )(9.81m / s 2 ) 1 kPa 1 kg 1N (100 cm) 2 L 2.0cm b) c) Pguage P Patm 104.0 101.33 2.67 kPa 0.0267 bar 1 atm Pabs 104.0 kPa 1.026 atm 101.33 kPa 14.7 psi 1.026 atm 15.09 psi 1 atm