Review of Thermodynamics - University of Alabama at Birmingham
advertisement

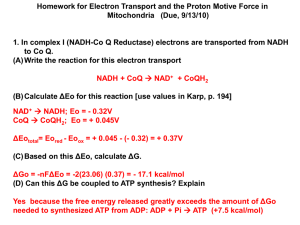
Thermodynamics of Biological Systems Champion Deivanayagam Center for Biophysical Sciences and Engineering University of Alabama at Birmingham. Outline: Laws of thermodynamics Enthalpy Entropy Gibbs free energy some examples What you need to know for your exam: 1. 2. 3. 4. 5. Definition of a thermodynamic system Three laws of Thermodynamics Definitions of Enthalpy, Entropy Gibbs Free energy ATP’s ionization states and its potential Energy: Energy is the capacity to do work 1. Kinetic Energy 2. Potential Energy • Kinetic energy is the form of energy expended by objects in motion • A resting object still possess energy in the form of potential energy Energy can be converted from one form into another Thermodynamics: A study of energy changes in systems: System: 1. Isolated 2. Closed 3. Open First law of thermodynamics: Energy is neither created nor destroyed; the energy of the universe is a constant The total internal energy of an isolated system in conserved. E = E2 – E1 = q + w q – heat absorbed by the system from surroundings w – work done on the system by the surroundings Mechanical work is defined as movement through some distance caused by the application of force Internal energy is independent of path and represents the present state of the system and is referred to as a State function Mechanical Work: At constant pressure work can be defined as w = -PV where V = V2 – V1 Work may occur in multiple forms: 1. Mechanical 2 . Electrical 3. magnetic 4. Chemical The calorie (cal, kcal) are traditional units Joule’s is the recommended SI unit. Table of important thermodynamic units and constants Enthalpy: H = E + PV In a constant pressure system (as in most biological systems) one can then define it as H = E + PV When you expand on this equation: H = q (simply put the heat energy of the system) Enthalpy changes can be measured using a calorimeter: For a system at equilibrium for any process where AB the standard enthalpy can be determined from the temperature dependence using: R- is the gas constant R= 8.314 J/mol . K Notice the ° sign: These are used to denote standard state: For solutes in a solution, the standard state is normally unit activity (simplified to 1M concentration) Protein denaturation: Study of temperature induced reversible denaturation of chymotrypsinogen At pH 3.0 T(K) Keq 324.4 326.6 0.041 0.12 327.5 0.27 329.0 0.68 330.7 1.9 332.0 333.8 5.0 21.0 van’t Hoff Plot Native state (N) Denatured state (D) Keq = [D] / [N] H° at any given temperature is the negative of the slope of the plot: H° = -[14.42]/[-0.027] x 10-3 = +533 kJ/mol Positive values for H° would be expected to break bonds and expose hydrophobic groups During the unfolding process and raise the energy of the protein in solution. Second law of thermodynamics: Every energy transfer increases the entropy (disorder) of the universe System tends to proceed from ordered states to disordered states Some definitions: Reversible: a process where transfer of energy happens in both directions Irreversible: a process where transfer of energy flows in one direction Equilibrium: A B (all naturally occurring process tend to equilibrium) Entropy: S = k ln W S = k ln Wfinal – k ln Winitial k- is the Boltzmann’s constant W – number of microstates Relationship between entropy and temperature dSreversible = dq/T Entropy changes measure the dispersal of energy in a process. Third law of thermodynamics: Entropy of any crystalline substance must approach zero as temperature approaches 0° K The absolute entropy can be calculated from this equation: Cp is the heat capacity, defined as the amount of heat 1 mole of it can store as the temperature of that substance is raised by 1 degree. For biological systems entropy changes are more useful than absolute entropies Gibb’s free energy ‘G’ Determines the direction of any reaction from the equation: G = H – TS For a constant pressure and temperature system (as most biological systems) then the Equation becomes easier to handle G = H - TS The enthalpy and entropy are now defined in one equation. G is negative for exergonic reactions (release energy in the form of work) is positive for endergonic reactions (absorbing energy in the form of work) Consider a reaction: A + B C + D [ C ][ D ] G G RT ln [ A ][ B ] At Equilibrium: G° = RT ln Keq and Keq = 10 - G°/2.3RT Example of chymotrypsinogen denaturation From the van’t Hoff plot we calculated H° = +533 kJ/mol At pH 3.0 T(K) 324.4 326.6 327.5 329.0 330.7 332.0 333.8 Keq 0.041 0.13 0.27 0.68 1.9 5.0 21.0 The equilibrium constant at 54.5 °C (327.5K) is 0.27 Then G° = (-8.314 J/mol·K) (327.5K) ln (0.27) = - 3.56 kJ/mol Similarly calculating S° = - (G - H°) / T = 1620 J/mol·K For a process to occur spontaneously the system must either give up energy (decrease H) or give up order (increase in S) or both In general for the process to be spontaneous: G must be negative The more negative the G value, the greater the amount of work the process can perform Exergonic reactions: G is negative and the reaction is spontaneous Example: Cellular respiration C6H12O6 + 6O2 6C02 + 6 H20 G = -686 kcal/mol (-2870 kJ/mol) (For each molecule of glucose broken 686 kcal energy is made available for work) Endergonic reactions: G is positive and requires large input of energy: Example: Photosynthesis where the energy is derived from the sun. Table: Variation of Reaction Spontaneity (Sign of G) with the signs of H and S. ATP: Adenosine triphosphate A cell does three kinds of work: 1. Mechanical work: beating of cilia, muscle contraction etc. 2. Transport work: Moving substances across membranes 3. Chemical work: Enabling non-spontaneous reactions to occur spontaneously e.g. Protein synthesis. The molecule that powers most kinds of work in the cell is ATP Energy is released when one or more phosphate groups are hydrolyzed ATP + H2O → ADP + Pi (G° = -35.7 kJ/mol) G° = RT ln Keq The activation energies for phosphoryl group-transfer reactions (200 to 400 kJ/mol) are substantially larger than the free energy of hydrolysis of ATP (30.5 kJ/mol). ΔGo` = -30.5 kJ/mole = -7.3 kcal/mole ΔG` = -52 kJ/mole = -12.4 kcal/mole Cellular conditions: [ADP][Pi] / [ATP] = 1/850 Ionization States of ATP • ATP has five dissociable protons • pKa values range from 0-1 to 6.95 • Free energy of hydrolysis of ATP is relatively constant from pH 1 to 6, but rises steeply at high pH • Since most biological reactions occur near pH 7, this variation is usually of little consequence The pH dependence of the free energy of hydrolysis of ATP. Because pH varies only slightly in biological environments, the effect on G is usually small. The free energy of hydrolysis of ATP as a function of total Mg2+ ion concentration at 38°C and pH 7.0. (Adapted from Gwynn, R. W., and Veech, R. L., 1973. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. Journal of Biological Chemistry 248:6966–6972.) The free energy of hydrolysis of ATP as a function of concentration at 38°C, pH 7.0. The plot follows the relationship described in Equation (3.36), with the concentrations [C] of ATP, ADP, and Pi assumed to be equal. What is the Daily Human Requirement for ATP? • The average adult human consumes approximately 11,700 kJ of food energy per day • Assuming thermodynamic efficiency of 50%, about 5860 kJ of this energy ends up in form of ATP • Assuming 50 kJ of energy required to synthesize one mole of ATP, the body must cycle through 5860/50 or 117 moles of ATP per day • This is equivalent to 65 kg of ATP per day • The typical adult human body contains 50 g of ATP/ADP • Thus each ATP molecule must be recycled nearly 1300 times per day Isothermal titration calorimetry Reference and experimental cell Heat energy required to maintain both of them At the same level is measured and integrated. This allows for the measurement of Kd, G and S ITC studies on A123 + VP3 regions 1 39 S 201 448 578 A1 A2 A3 A1 A2 A3 V-region 828 840 P1 P2 960 P3 V-region P1 P2 P3 1486 1561 WMC Time (min) 0 Large release of Heat energy indicates Ordering of structures Hydrogen bonding The Kd and the energy released indicate that the interaction between these two regions is strong. 20 30 40 50 60 70 µcal/sec -8 -10 Titration of 12 M VP3 by 10 L x 25 injections of 96 M A1-3 -12 o at 25 C kcal/mole of injectant 1:1 Stoichiometric ratio (N=0.946 ± 0.006) and Kd ~ 40 nm 10 -14 0 -50 -100 -150 N Ka 0.946 + 0.006 7 -1 (2.6 + 0.4) x 10 M G H T S -10.1 kcal/mol (-212 + 2) kcal/mol -202 kcal/mol -200 0.0 0.5 1.0 Molar Ratio 1.5 What happens in a cell if G = 0 ?