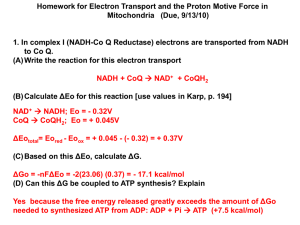
A little thermodynamics
advertisement

CH339K A little thermodynamics (which is probably more than anybody wants) Thermodynamics (Briefly) • Systems est divisa in partes tres – Open • Exchange energy and matter – Closed • Exchange energy only – Isolated • Exchange nothing More Thermodynamics • Energy can be exchanged as heat (q) or work (w) • By convention: – q > 0: heat has been gained by the system from the surroundings – q < 0: heat has been lost by the system to the surroundings – w > 0: work has been done by the system on the surroundings – w < 0: work has been done on the system by the surroundings First Law of Thermo • E SYSTEM = q – w or, alternatively, q = E + w First law of Thermo (cont.) Example: Oxidation of a Fatty Acid (Palmitic): C16H32O2 + 23O2 (g) 16CO2 (g) + 16H2O (l) • Under Constant Volume: q = -9941.4 kJ/mol. • Under Constant Pressure: q = -9958.7 kJ/mol First Law of Thermo (cont.) • Why the difference? • Under Constant Volume, q = E + w = -9941.4 kJ/mol + 0 = -9941.4 kJ/mol • Under Constant Pressure, W is not 0! Used 23 moles O2, only produced 16 moles CO2 W = PΔV ΔV = ΔnRT/P W = ΔnRT = (-7 mol)(8.314 J/Kmol)(298 K) = -17.3 kJ q = -9941.4 kJ/mol + (-17.3 kJ/mol) = -9958.7 kJ/mol Enthalpy • Technically speaking, most cells operate under constant pressure conditions • Practically, there’s not much difference most of the time • Enthalpy (H) is defined as: H = E + PV or H = E + PV • If H > 0, heat is flowing from the surroundings to the system and the process is endothermic • if H < 0, heat is being given off, and the process is exothermic. • Many spontaneous processes are exothermic, but not all Endothermic but spontaneous • Ammonium Nitrate spontaneously dissolves in water to the tune of about 2 kg/liter • Ammonium nitrate has a Hsolution of +25.7 kJ/mol • Remember positive enthalpy = endothermic • This is the basis of instant cold packs Second law of Thermo • Any spontaneous process must be accompanied by a net increase in entropy (S). • What the heck is entropy? • Entropy is a measure of the “disorderliness” of a system (and/or the surroundings). • What the heck does that mean? • Better, it is a measure of the number of states that a system can occupy. • Huh?...let me explain Entropy S = k x ln(W) where • W is the number of possible states • k is Boltzmann’s constant, = R/N Two states of 5 “atoms” in 50 possible “slots.” State 1… State 2… etc… X X X X X X X X X X What happens if the volume increases? K K K K K Adding volume increases the number of “slots,” therefore increasing W, the number of states, thereby increasing entropy. • We can quantify that: – Number of atoms dissolved = Na – Number of original slots = no – Number of original states = Wo – Number of final slots = nf – Number of final states = Wf Wo = no (no 1)(no 2)...(n o Na) Wf = nf (nf 1)(nf 2)...(nf Na) • Since Na << Wo and Na << Wf (dilute solution), then: n o Na n oand n f Na n f • So we can simplify the top equations to: Wo = n Na o and Wf = n Na f • Okay, so what (quantitatively) is the change in entropy from increasing the volume? S = Sf - So • Substituting and solving: S = k ln(Wf ) k ln(Wo ) Wf S = k ln Wo n fNa S = k ln Na no nf S = k ln no Na nf S = Na k ln no So S is logarithmically related to the change in the number of “slots.” • Let’s make the assumption that we are dealing with 1 mole (i.e. N atoms) of solute dissolved in a large volume of water. • Since Boltzmann’s constant (k) = R/N, our equation resolves to: nf S = R ln no • Since the number of “slots” is directly related to the volume: Vf S = R ln Vo • And since the concentration is inversely related to the volume: Co S = R ln Cf Entropy (cont.) • Entropy change tells us whether a reaction is spontaneous, but… • Entropy can increase in the System, the Surroundings, or both, as long as the total is positive. • Can’t directly measure the entropy of the surroundings. • HOWEVER, the change in enthalpy of the system is an indirect measure of the change in entropy of the surroundings – an exothermic reaction contributes heat (disorder) to the universe. Gibbs Free Energy • We can coin a term called the Free Energy (G) of the system which tells us the directionality of a reaction. G = H – TS ΔG = ΔH - T ΔS If ΔG < 0, free energy is lost exergonic – forward rxn favored. If ΔG > 0, free energy is gained endergonic – reverse rxn favored. Different ΔG’s • ΔG is the change in free energy for a reaction under some set of real conditions. • ΔGo is the change in free energy for a reaction under standard conditions (all reactants 1M) • ΔGo’ is the change of free energy for a reaction with all reactants at 1M and pH 7. Partial Molar free Energies • The free energy of a mixture of stuff is equal to the total free energies of all its components • The free energy contribution of each component is the partial molar free energy: Gx Gx0 RT ln[G] • Where: Gx0 thestandardfree energyof thecomponent [Gx ] theactivity of thecomponentin themixtureor solution • In dilute (i.e. biochemical) solutions, • the activity of a solute is its concentration • The activity of the solvent is 1 Free Energy and Chemical Equilibrium Take a simple reaction: A+B⇌C+D Then we can figure the Free Energy Change: G Gproducts Greactants G GoC RTlnC GoD RTlnD - GoA - RTlnA - GoB - RTlnB Rearranging G G oC G oD G oA G oB RTlnC RTlnD - RTlnA - RTlnB Combining G G o RTlnC lnD - lnA - lnB Factoring CD G G RT ln AB o Freee Energy and Equilibrium (cont.) CD G G o RT ln AB Hang on a second! [A][B] is the product of the reactant concentrations [C][D] is the product of the product concentrations Products G G RTln Reactants o Remembering Freshman Chem, we have a word for that ratio. CD K eq AB Free Energy and Equilibrium (cont.) SO: ΔGo for a reaction is related to the equilibrium constant for that reaction. ΔGo = -RTlnKeq Or Keq = e-ΔGo/RT Note: things profs highlight with colored arrows are probably worth remembering If you know one, you can determine the other. Real Free Energy of a Reaction As derived 2 slides previously: G is related to Go’, adjusted for the concentration of the reactants: [Products] ΔG ΔG ' RT ln [Reactants] o Example: Glucose-6-Phosphate ⇄ Glucose + Pi ∆Go’ = -13.8 kJ/mol At 100 μM Glucose-6-Phosphate 5 mM Phosphate 10 mM Glucose [Glucose][ Pi] ΔG ΔG ' RT ln [Glucose 6 Phosphate] o (.01M)(.005M) ΔG 13800J/mol 8.315J/Kmol 310K ln (.0001M) ΔG 13800J/mol (1787J/mol) 15587J/mol Measuring H, S, and G We know ΔG = ΔH - T ΔS And ΔGo = -RTlnKeq So ΔH - T ΔS = -RTlnKeq Or o H 1 S lnK eq R T R o Measuring H, S, and G H 1 S lnK eq R T R o • • • • o This is the van’t Hoff Equation You can control T You can measure Keq If you plot ln(Keq) versus 1/T, you get a line – Slope = -ΔHo/R – Y-intercept = ΔSo/R Van’t Hoff Plot 1.00 0.90 y = -902.09x + 3.6084 0.80 0.70 ln(Keq) 0.60 0.50 0.40 0.30 0.20 0.10 0.00 0.0031 0.0032 0.0033 0.0034 0.0035 0.0036 0.0037 1/T (K-1) ΔHo = -902.1* 8.315 = -7500 J/mol ΔSo = +3.61 * 8.315 = 30 J/Kmol Why the big Go’ for Hydrolyzing Phosphoanhydrides? • Electrostatic repulsion betwixt negative charges • Resonance stabilization of products • pH effects pH Effects – o G vs. o G ’ Products G G o RT ln Reactants ADPPiH G G RT ln ATPH 2O H ADPPi o RT ln G G RT ln H 2O ATP o At pH 7, H 10-7 M ADPPi RT ln107 G o ' G o RT ln ATP ADPPi 41.5 kJ G o ' G o RT ln ATP mol G in kcal/mol) WOW! Cellular Gs are not Go’ s Go’ for hydrolysis of ATP is about -31 kJ/mol Cellular conditions are not standard, however: In a human erythrocyte, [ATP]≈2.25 mM, [ADP] ≈0.25 mM, [PO4] ≈1.65 mM [ ADP][Pi] [ ATP] kJ J (.00025M )(.00165M ) 31 8.315 298K ln m ol K m ol (.00225M ) kJ kJ kJ 31 (21 ) 52 m ol m ol m ol GHyd G o ' RT ln GHyd GHyd Unfavorable Reactions can be Subsidized with Favorable Ones Hydrolysis of Thioesters can also provide a lot of free energy Acetyl Coenzyme A Sample Go’Hydrolysis